Abstract
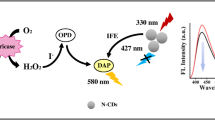
A dual-read detection system is described for non-enzymatic and non-aggregation based analysis of uric acid (UA). Silver triangular nanoprisms (AgTNPs) were used as colorimetric probes, while the reduction in the fluorescence of nitrogen-doped carbon quantum dots (N-CQDs) served as the fluorometric readout. The absorption band of the AgTNPs overlaps the emission band of N-CQDs (with a peak at 440 nm). Therefore, fluorescence is reduced owing to an inner filter effect. The AgTNPs are etched if exposed to H2O2, and round nanodiscs are formed. In the presence of UA, etching of the AgTNPs is suppressed because the facets of the AgTNPs are coated with UA. The absorbance, best measured at 683 nm, increases with the concentration of the pre-added UA. The colorimetric assay works in the 0.1–45 μM UA concentration range, and the fluorometric assay between 1 and 42 μM of UA. The respective detection limits are 50 and 200 nM, respectively. The probe can be used for direct visualization of UA. The method was successfully applied to the determination of UA in urine samples.

The fluorescence of nitrogen-doped carbon quantum dots (N-CQDs) is quenched by AgTNPs (silver triangular nanoprisms). As the AgTNPs are etched by H2O2, fluorescence recovers in the system after H2O2 is added, and also undergoes a color change. Uric acid (UA) protects the AgTNPs from etching because the facets of the AgTNPs are coated with UA. The fluorescence of N-CQDs decreases. Thus, a dual-read probe is developed for determination of UA.





Similar content being viewed by others
References
Álvarez-Lario B, Macarrón-Vicente J (2010) Uric acid and evolution. Rheumatology 49(11):2010–2015
YC Luo, JS Do, CC Liu (2006) An amperometric uric acid biosensor based on modified Ir-C electrode. Biosens Bioelectron 22(4):482–488
Erden PE, Kılıç E (2013) A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 107(107C):312–323
Dai X, Fang X, Zhang C, Xu R, Xu B (2007) Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference method. J Chromotogr B 857(2):287–295
Galbán J, Andreu Y, Almenara MJ, De MS, Castillo JR (2001) Direct determination of uric acid in serum by a fluorometric-enzymatic method based on uricase. Talanta 54(5):847–854
Ali SMU, Alvi NH, Ibupoto Z, Nur O, Willander M, Danielsson B (2011) Selective potentiometric determination of uric acid with uricase immobilized on ZnO nanowires. Sensor Actuat B-Chem 152(2):241–247
Chen X, Wu G, Cai Z, Oyama M, Chen X (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181(7):689–705
Jin D, Seo MH, Huy BT, Pham QT, Conte ML, Thangadurai D, Lee YI (2016) Quantitative determination of uric acid using CdTe nanoparticles as fluorescence probes. Biosens Bioelectron 77:359–365
Wu D, Lu H-F, Xie H, Wu J, Wang C-M, Zhang Q-L (2015) Uricase-stimulated etching of silver nanoprisms for highly selective and sensitive colorimetric detection of uric acid in human serum. Sensor Actuat B-Chem 221:1433–1440
Cantale V, Simeone FC, Gambari R, Rampi MA (2011) Gold nano-islands on FTO as plasmonic nanostructures for biosensors. Sensor Actuat B-Chem 152(2):206–213
Rao H, Ge H, Wang X, Zhang Z, Liu X, Yang Y, Liu Y, Liu W, Zou P, Wang Y (2017) Colorimetric and fluorometric detection of protamine by using a dual-mode probe consisting of carbon quantum dots and gold nanoparticles. Microchim Acta 184(8):1–9
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interfac 145(1):83–96
Sherry LJ, Jin R, Mirkin CA, And GCS, Duyne RPV (2006) Localized surface plasmon resonance spectroscopy of single silver triangular Nanoprisms. Nano Lett 6(9):2060–2065
Zhang YL, Wang L, Zhang HC, Liu Y, Wang HY, Kang ZH, Lee ST (2013) Graphitic carbon quantum dots as a fluorescent sensing platform for highly efficient detection of Fe3+ ions. RSC Adv 3(11):3733–3738
Dai H, Shi Y, Wang Y, Sun Y, Hu J, Ni P, Li Z (2014) A carbon dot based biosensor for melamine detection by fluorescence resonance energy transfer. Sensor Actuat B-Chem 202(19):201–208
Zhao D, Chen C, Sun J, Yang X (2016) Carbon dots-assisted colorimetric and fluorometric dual-mode protocol for acetylcholinesterase activity and inhibitors screening based on the inner filter effect of silver nanoparticles. Analyst 141(11):3280–3288
Gao X, Du C, Zhuang Z, Chen W (2016) Carbon quantum dot-based nanoprobes for metal ion detection. J Mater Chem C 4(29):6927–6945
Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, Zhang K, Sun H, Wang H, Yang B (2013) Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem 52(14):3953–3957
Fan Y, Cheng H, Zhou C, Xie X, Liu Y, Dai L, Zhang J, Qu L (2012) Honeycomb architecture of carbon quantum dots: a new efficient substrate to support gold for stronger SERS. Nano 4(5):1776–1781
Wang Z, Fan Z (2018) Cu2+ modulated nitrogen-doped grapheme quantum dots as a turn-off/on fluorescence sensor for the selective detection of histidine in biological fluid. Spectrochim Acta A 189:195–201
Liu Z, Gong Y, Fan Z (2016) Cysteine detection using a high-fluorescence sensor based on a nitrogen-doped graphene quantum dot–mercury(II) system. J Lumin 175:129–134
Sun Y, Brian Mayers A, Xia Y (2003) Transformation of silver nanospheres into nanobelts and triangular Nanoplates through a thermal process. Nano Lett 3(5):675–679
Corro G, Vidal E, Cebada S, Pal U, Bañuelos F, Vargas D, Guilleminot E (2017) Electronic state of silver in Ag/SiO2 and Ag/ZnO catalysts and its effect on diesel particulate matter oxidation: an XPS study. Appl Catal B Environ 216:1–10
Yu X, Liu J, Yu Y, Zuo S, Li B (2014) Preparation and visible light photocatalytic activity of carbon quantum dots/TiO 2 nanosheet composites. Carbon 68(3):718–724
Xiao Z, Loughlin F, George GN, Howlett GJ, Wedd AG (2004) C-terminal domain of the membrane copper transporter Ctr1 from Saccharomyces cerevisiae binds four Cu(I) ions as a cuprous-thiolate polynuclear cluster: sub-femtomolar Cu(I) affinity of three proteins involved in copper trafficking. J Am Chem Soc 126(10):3081–3090
Shao N, Zhang Y, Cheung S, Yang R, Chan W, Mo T, Li K, Liu F (2005) Copper ion-selective fluorescent sensor based on the inner filter effect using a spiropyran derivative. Anal Chem 77(22):7294–7303
Fang A, Wu Q, Lu Q, Chen H, Li H, Liu M, Zhang Y, Yao S (2016) Upconversion ratiometric fluorescence and colorimetric dual-readout assay for uric acid. Biosens Bioelectron 86:664–670
Millstone JE, Hurst SJ, Métraux GS, Cutler JI, Mirkin CA (2009) Colloidal gold and silver triangular nanoprisms. Small 5(6):646–664
Tan K, Yang G, Chen H, Shen P, Huang Y, Xia Y (2014) Facet dependent binding and etching: ultra-sensitive colorimetric visualization of blood uric acid by unmodified silver nanoprisms. Biosens Bioelectron 59(13):227–232
Zhao Y, Yan X, Kang Z, Lin P, Fang X, Lei Y, Ma S, Zhang Y (2013) Highly sensitive uric acid biosensor based on individual zinc oxide micro/nanowires. Microchim Acta 180(9-10):759–766
Wang H, Lu Q, Hou Y, Liu Y, Zhang Y (2016) High fluorescence S, N co-doped carbon dots as an ultra-sensitive fluorescent probe for the determination of uric acid. Talanta 155:62–69
Zhou Y, Ling B, Chen H, Wang L (2018) Mn2+ -doped NaYF4:Yb,Er upconversion nanoparticles for detection of uric acid based on the Fenton reaction. Talanta 180:120–126
Kong R-M, Yang A, Wang Q, Wang Y, Ma L, Qu F (2017) Uricase based fluorometric determination of uric acid based on the use of graphene quantum dot@silver core-shell nanocomposites. Microchim Acta 185(1):63
da Cruz FS, Paula F d S, Franco DL, dos Santos WTP, Ferreira LF (2017) Electrochemical detection of uric acid using graphite screen-printed electrodes modified with Prussian blue/poly(4-aminosalicylic acid)/Uricase. J Electroanal Chem 806:172–179
Wang Y (2011) The electrochemistry of uric acid at a gold electrode modified with L-cysteine, and its application to sensing uric urine. Microchim Acta 172(3):419–424
Yang Y, Jo A, Lee Y, Lee C (2018) Electrodeposited nanoporous ruthenium oxide for simultaneous quantification of ascorbic acid and uric acid using chronoamperometry at two different potentials. Sensor Actuat B-Chem 255:316–324
Rana L, Gupta R, Tomar M, Gupta V (2018) Highly sensitive Love wave acoustic biosensor for uric acid. Sensor Actuat B-Chem 261:169–177
Acknowledgments
This work was supported by a grant from the Two-way Support Programs of Sichuan Agricultural University (Project No.03572228) and the Education Department of Sichuan Provincial, PR China (Grant No. 16ZA0039).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interest.
Additional information
The authors wish it to be known that, in their opinions, Yanying Wang and Yan Yang should be regarded as joint First Authors.
Electronic supplementary material
ESM 1
(DOC 13035 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Yang, Y., Liu, W. et al. Colorimetric and fluorometric determination of uric acid based on the use of nitrogen-doped carbon quantum dots and silver triangular nanoprisms. Microchim Acta 185, 281 (2018). https://doi.org/10.1007/s00604-018-2814-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2814-6