Abstract
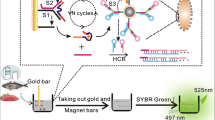
The authors describe an enzyme-free aptamer-based assay for the determination of the model antibiotic kanamycin (Kana). The method is making use of (a) microfluidic chip electrophoresis; (b) a stirring bar carrying a gold-labeled aptamer probe, and (c) the hybridization chain reaction (HCR) for signal amplification. Firstly, a stirring bar (length: 1 cm; diameter: 0.2 mm) was modified with a large amount of duplex DNA and then hybridized with aptamer and its partially complementary chains (cDNA). In the presence of Kana, the binding between the Kana and aptamer unwinds the duplex structures and releases a corresponding amount of cDNA into the supernatant. The released cDNA triggers the HCR in the presence of H1 and H2 DNA hairpin to produce a large amount of duplex DNA chains with different lengths. At the same time, the amounts of H1 and H2 are reduced. The decreased signal of H1/H2 after several HCR cycles can be used to quantify kana in the 1 pg·mL−1 to 10 ng·mL−1, with a detection limit of 0.29 pg·mL−1. The signal is generated by reading the fluorescence, best at excitation/emission maxima of 470/525 nm. The whole detection process takes 3 min only. The assay was employed to the detection of Kana in spiked milk and fish samples. Results are consistent with those of an enzyme linked immunosorbent assay. The assay has high throughput, high selectivity, and high amplification capability.

Schematic of a stirring bar functionalized with gold-labeled aptamer acting as the capture probe. It can capture the target and release primer simultaneously. The primer triggers the hybridization chain reaction inducing the consumption of H1 and H2. After a certain reaction time, the mixture is injected into the MCE platform for microfluidic electrophoretic separation and fluorometric detection.





Similar content being viewed by others
References
Chen Z, Li Q, Sun Q, Chen H, Wang X, Li N, Yin M, Xie Y, Li H, Tang B (2012) Simultaneous determination of reactive oxygen and nitrogen species in mitochondrial compartments of apoptotic HepG2 cells and PC12 cells based on microchip electrophoresis–laser-induced fluorescence. Anal Chem 84(11):4687–4694
Lin Y, Trouillon R, Safina G, Ewing AG (2011) Chemical analysis of single cells. Anal Chem 83(12):4369–4392
Zhang X, Li Q, Chen Z, Li H, Xu K, Zhang L, Tang B (2011) Electrokinetic gated injection-based microfluidic system for quantitative analysis of hydrogen peroxide in individual HepG2 cells. Lab Chip 11(6):1144–1150
Phillips KS, Lai HH, Johnson E, Sims CE, Allbritton NL (2011) Continuous analysis of dye-loaded, single cells on a microfluidic chip. Lab Chip 11(7):1333–1341
Hou J, Zhang H, Yang Q, Li M, Song Y, Jiang L (2014) Bio-inspired photonic-crystal microchip for fluorescent ultratrace detection. Angew Chem 53(23):5791
Zhu G, Song Q, Liu W, Yan X, Xiao J, Chen C (2017) A gold nanoparticle-modified indium tin oxide microelectrode for in-channel amperometric detection in dual-channel microchip electrophoresis. Anal Methods 9(29):4319–4326
Meng HL, Chen GH, Guo X, Chen P, Cai QH, Tian YF (2014) Determination of five quinolone antibiotic residues in foods by micellar electrokinetic capillary chromatography with quantum dot indirect laser-induced fluorescence. Anal Bioanal Chem 406(13):3201–3208
de Kort BJ, de Jong GJ, Somsen GW (2013) Native fluorescence detection of biomolecular and pharmaceutical compounds in capillary electrophoresis: detector designs, performance and applications: a review. Anal Chim Acta 766:13–33
Tseng HM, Li Y, Barrett DA (2010) Bioanalytical applications of capillary electrophoresis with laser-induced native fluorescence detection. Bioanalysis 2(9):1641–1653
Schwarzkopf F, Scholl T, Ohla S, Belder D (2014) Improving sensitivity in microchip electrophoresis coupled to ESI-MS/MS on the example of a cardiac drug mixture. Electrophoresis 35(12–13):1880–1886
Cruz-Aguado JA, Penner G (2008) Determination of ochratoxin a with a DNA aptamer. J Agric Food Chem 56(22):10456–10461
Song S, Wang L, Li J, Fan C, Zhao J (2008) Aptamer-based biosensors. TrAC Trends Anal Chem 27(2):108–117
Lan L, Yao Y, Ping J, Ying Y (2017) Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens Bioelectron
Zhou L, Gan N, Zhou Y, Li T, Cao Y, Chen Y (2017) A label-free and universal platform for antibiotics detection based on microchip electrophoresis using aptamer probes. Talanta 167:544–549
Wang Y, Gan N, Zhou Y, Li TH, Hu FT, Cao YT, Chen YJ (2017) Novel label-free and high-throughput microchip electrophoresis platform for multiplex antibiotic residues detection based on aptamer probes and target catalyzed hairpin assembly for signal amplification. Biosens Bioelectron 97:100–106
Rebe Raz S, Bremer MG, Haasnoot W, Norde W (2009) Label-free and multiplex detection of antibiotic residues in milk using imaging surface plasmon resonance-based immunosensor. Anal Chem 81(18):7743–7749
Bai Y, Song M, Cui Y, Shi C, Wang D, Paoli GC, Shi X (2013) A rapid method for the detection of foodborne pathogens by extraction of a trace amount of DNA from raw milk based on amino-modified silica-coated magnetic nanoparticles and polymerase chain reaction. Anal Chim Acta 787:93–101
Hao L, Gu H, Duan N, Wu S, Ma X, Xia Y, Tao Z, Wang Z (2017) An enhanced chemiluminescence resonance energy transfer aptasensor based on rolling circle amplification and WS2 nanosheet for Staphylococcus Aureus detection. Anal Chim Acta 959:83–90
Cheng Y, Du Q, Wang L, Jia H, Li Z (2012) Fluorescently cationic conjugated polymer as an indicator of ligase chain reaction for sensitive and homogeneous detection of single nucleotide polymorphism. Anal Chem 84(8):3739–3744
Chen C, Li N, Lan J, Ji X, He Z (2016) A label-free colorimetric platform for DNA via target-catalyzed hairpin assembly and the peroxidase-like catalytic of graphene/au-NPs hybrids. Anal Chim Acta 902:154–159
Liu H, Bei X, Xia Q, Fu Y, Zhang S, Liu M, Fan K, Zhang M, Yang Y (2015) Enzyme-free electrochemical detection of microRNA-21 using immobilized hairpin probes and a target-triggered hybridization chain reaction amplification strategy. Microchim Acta 183(1):297–304
Wang B, Zhou X, Yao D, Sun X, He M, Wang X, Yin X, Liang H (2017) Contribution of gold nanoparticles to the catalytic DNA strand displacement in leakage reduction and signal amplification. Chem Commun
Bi S, Yue S, Zhang S (2017) Hybridization chain reaction: a versatile molecular tool for biosensing, bioimaging, and biomedicine. Chem Soc Rev 46(14):4281
Liu H, Gan N, Chen Y, Li T, Cao Y (2017) Three dimensional M× N type aptamer-functionalized solid-phase micro extraction fibers array for selectively sorptive extraction of multiple antibiotic residues in milk. RSC Adv 7(12):6800–6808
Qin Y, Zhang L, Li S, Zhao J, Huang Y, Zhao S, Liu YM (2016) A microchip electrophoresis-based fluorescence signal amplification strategy for highly sensitive detection of biomolecules. Chem Commun 53(2):455–458
Song K-M, Cho M, Jo H, Min K, Jeon SH, Kim T, Han MS, JK K, Ban C (2011) Gold nanoparticle-based colorimetric detection of kanamycin using a DNA aptamer. Anal Biochem 415(2):175–181
Wang L, Wang E (2004) Direct electron transfer between cytochrome c and a gold nanoparticles modified electrode. Electrochem Commun 6(1):49–54
Takino M, Daishima S, Nakahara T (2003) Determination of chloramphenicol residues in fish meats by liquid chromatography–atmospheric pressure photoionization mass spectrometry. J Chromatogr A 1011 (1):67–75
Song H-Y, Kang T-F, Li N-N, L-P L, Cheng S-Y (2016) Highly sensitive voltammetric determination of kanamycin based on aptamer sensor for signal amplification. Anal Methods 8(16):3366–3372
Han C, Li R, Li H, Liu S, Xu C, Wang J, Wang Y, Huang J (2017) Ultrasensitive voltammetric determination of kanamycin using a target-triggered cascade enzymatic recycling couple along with DNAzyme amplification. Microchim Acta 184(8):1–8
Ramezani M, Danesh NM, Lavaee P, Abnous K, Taghdisi SM (2016) A selective and sensitive fluorescent aptasensor for detection of kanamycin based on catalytic recycling activity of exonuclease III and gold nanoparticles. Sensors Actuators B Chem 222(1):1–7
Liao QG, Wei BH, Luo LG (2016) Aptamer based fluorometric determination of kanamycin using double-stranded DNA and carbon nanotubes. Microchim Acta:1–6
Liu C, Lu C, Tang Z, Chen X, Wang G, Sun F (2015) Aptamer-functionalized magnetic nanoparticles for simultaneous fluorometric determination of oxytetracycline and kanamycin. Microchim Acta 182(15–16):2567–2575
Lai C, Liu X, Qin L, Zhang C, Zeng G, Huang D, Cheng M, Xu P, Yi H, Huang D (2017) Chitosan-wrapped gold nanoparticles for hydrogen-bonding recognition and colorimetric determination of the antibiotic kanamycin. Microchim Acta 184(19):1–9
Acknowledgments
This work was supported by National Key R&D Program of China (No.2016YFD0401502), the National Natural Science Foundation of China (No.51403110), the Natural Science Foundation of Zhejiang and Ningbo (LY17C200007, 2017C33004, 2017C37023, Y18B070008, LY15B050002, LY16B050003, 2017A610225, 2016A610084), and K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 1412 kb)
Rights and permissions
About this article
Cite this article
Zhang, K., Gan, N., Hu, F. et al. Microfluidic electrophoretic non-enzymatic kanamycin assay making use of a stirring bar functionalized with gold-labeled aptamer, of a fluorescent DNA probe, and of signal amplification via hybridization chain reaction. Microchim Acta 185, 181 (2018). https://doi.org/10.1007/s00604-017-2635-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2635-z