Abstract
Purpose
The aim of this study was to examine the quality of data from the National Clinical Database (NCD) via a comparison with regional government report data and medical charts.
Methods
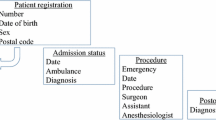
A total of 1,165,790 surgical cases from 3007 hospitals were registered in the NCD in 2011. To evaluate the NCD’s data coverage, we retrieved regional government report data for specified lung and esophageal surgeries and compared the number with registered cases in the NCD for corresponding procedures. We also randomly selected 21 sites for on-site data verification of eight demographic and surgical data components to assess the accuracy of data entry.
Results
The numbers of patients registered in the NCD and regional government report were 46,143 and 48,716, respectively, for lung surgeries and 7494 and 8399, respectively, for esophageal surgeries, leading to estimated coverages of 94.7% for lung surgeries and 89.2% for esophageal surgeries. According to on-site verification of 609 cases at 18 sites, the overall agreement between the NCD data components and medical charts was 97.8%.
Conclusion
Approximately, 90–95% of the specified lung surgeries and esophageal surgeries performed in Japan were registered in the NCD in 2011. The NCD data were accurate relative to medical charts.

Similar content being viewed by others
References
D’Agostino RS, Jacobs JP, Badhwar V, Paone G, Rankin JS, Han JM, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2017 update on outcomes and quality. Ann Thorac Surg. 2017;103:18–24.
Cohen ME, Liu Y, Ko CY, Hall BL. Improved surgical outcomes for ACS NSQIP hospitals over time: evaluation of hospital cohorts with up to 8 years of participation. Ann Surg. 2016;263:267–73.
Miyata H, Gotoh M, Hashimoto H, Motomura N, Murakami A, Tomotaki A, et al. Challenges and prospects of a clinical database linked to the board certification system. Surg Today. 2014;44:1991–9.
Saze Z, Miyata H, Konno H, Gotoh M, Anazawa T, Tomotaki A, et al. Risk models of operative morbidities in 16,930 critically ill surgical patients based on a Japanese nationwide database. Medicine (Baltimore). 2015;94:e1224.
Yokoo H, Miyata H, Konno H, Taketomi A, Kakisaka T, Hirahara N, et al. Models predicting the risks of six life-threatening morbidities and bile leakage in 14,970 hepatectomy patients registered in the National Clinical Database of Japan. Medicine (Baltimore). 2016;95:e5466.
Kurita N, Miyata H, Gotoh M, Shimada M, Imura S, Kimura W, et al. Risk model for distal gastrectomy when treating gastric cancer on the basis of data from 33,917 Japanese patients collected using a nationwide web-based data entry system. Ann Surg. 2015;262:295–303.
Gotoh M, Miyata H, Hashimoto H, Wakabayashi G, Konno H, Miyakawa S, et al. National Clinical Database feedback implementation for quality improvement of cancer treatment in Japan: from good to great through transparency. Surg Today. 2016;46:38–47.
Shroyer AL, Edwards FH, Grover FL. Updates to the data quality review program: the Society of Thoracic Surgeons Adult Cardiac National Database. Ann Thorac Surg. 1998;65:1494–7.
Grover FL, Shroyer AL, Edwards FH, Pae WE Jr, Ferguson TB Jr, Gay WA Jr, Clark RE. Data quality review program: the Society of Thoracic Surgeons Adult Cardiac National Database. Ann Thorac Surg. 1996;62:1229–31.
Shiloach M, Frencher SK Jr, Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16.
Maruszewski B, Lacour-Gayet F, Monro JL, Keogh BE, Tobota Z, Kansy A. An attempt at data verification in the EACTS Congenital Database. Eur J Cardiothorac Surg. 2005;28:400–4.
Tomotaki A, Miyata H, Hashimoto H, Murakami A, Ono M. Results of data verification of the Japan Congenital Cardiovascular Database, 2008 to 2009. World J Pediatr Congenit Heart Surg. 2014;5:47–53.
Elfström J, Stubberöd A, Troeng T. Patients not included in medical audit have a worse outcome than those included. Int J Qual Health Care. 1996;8:153–7.
Ministry of Health, Labor and Welfare. Survey of medical institutions of 2013. http://www.mhlw.go.jp/toukei/saikin/hw/iryosd/13/. Accessed 1 May 2017.
Kanto-Shinetsu Regional Bureau of Health and Welfare (in Japanese). https://kouseikyoku.mhlw.go.jp/kantoshinetsu/iryo_shido/sisetukijyunntodokede.html. Accessed 26 Oct 2017.
Miyata H, Tomotaki A, Okubo S, Motomura N, Murakami A, Kiuchi T, et al. Quality improvement initiative based on National Clinical Database (2) to enhance reliability and neutrality of quality assessment. Surg Ther. 2011;104:381–6.
The North American Association of Central Cancer Registries. https://www.naaccr.org/certification-criteria/. Accessed 26 Oct 2017.
Allemani C, Harewood R, Johnson CJ, Carreira H, Spika D, Bonaventure A, Ward K, Weir HK, Coleman MP. Population-based cancer survival in the United States: data, quality control, and statistical methods. Cancer. 2017;123(Suppl 24):4982–93.
Østgård LS, Nørgaard JM, Raaschou-Jensen KK, Pedersen RS, Rønnov-Jessen D, Pedersen PT, Dufva IH, Marcher CW, Nielsen OJ, Severinsen MT, Friis LS. The Danish National Acute Leukemia Registry. Clin Epidemiol. 2016;8:553–60.
Allepuz A, Serra-Sutton V, Martínez O, Tebé C, Nardi J, Portabella F, Espallargues M, en nombre del Registro de Artroplastias de Cataluña (RACat). Arthroplasty registers as post-marketing surveillance systems: the Catalan Arthroplasty Register. Rev Esp Cir Ortop Traumatol. 2013;57(1):27–37.
Vikholm P, Ivert T, Nilsson J, Holmgren A, Freter W, Ternström L, Ghaidan H, Sartipy U, Olsson C, Granfeldt H, Ragnarsson S, Friberg Ö. Validity of the Swedish Cardiac Surgery Registry. Interact Cardiovasc Thorac Surg. 2018. https://doi.org/10.1093/icvts/ivy030.
Linder G, Lindblad M, Djerf P, Elbe P, Johansson J, Lundell L, Hedberg J. Validation of data quality in the Swedish National Register for Oesophageal and Gastric Cancer. Br J Surg. 2016;103(10):1326–35.
Ministry of Health, Labor and Welfare. Outline of 2010 revision of medical fee (in Japanese). http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryouhoken/iryouhoken12/index.html. Accessed 26 Oct 2017.
Takahashi A, Kumamaru H, Tomotaki A, Matsumura G, Fukuchi E, Hirata Y, Murakami A, Hashimoto H, Ono M, Miyata H. Verification of data accuracy in Japan Congenital Cardiovascular Surgery Database including its postprocedural complication reports. World J Pediatr Congenit Heart Surg. 2018;9:150–6.
Acknowledgements
The authors thank all of the staff and academic societies involved in the National Clinical Database. AT, HK, and HM are affiliated with the Department of Healthcare Quality Assessment at the University of Tokyo, which is a social collaboration department supported by the National Clinical Database, Johnson & Johnson K.K., and Nipro corporation. The authors have no other conflicts of interest to declare. The data verification activities were supported by the National Clinical Database.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tomotaki, A., Kumamaru, H., Hashimoto, H. et al. Evaluating the quality of data from the Japanese National Clinical Database 2011 via a comparison with regional government report data and medical charts. Surg Today 49, 65–71 (2019). https://doi.org/10.1007/s00595-018-1700-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-018-1700-5