Abstract
Purpose
A triplet regimen of docetaxel, cisplatin, and S-1(DCS) is highly effective against metastatic gastric cancer. We performed this study to clarify the safety and efficacy of surgical resection in patients with initially unresectable gastric cancer, after down-staging or disease control was achieved by DCS chemotherapy.
Methods
The subjects of this retrospective study were 31 consecutive patients with initially unresectable gastric cancer, who underwent surgical resection between October, 2006 and December, 2012, after down-staging or disease control was achieved by DCS chemotherapy. We evaluated the clinicopathological factors and clinical outcomes and assessed radiographic response based on the RECIST criteria, not by central review.
Result
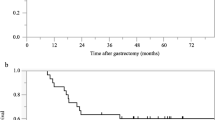
Before DCS chemotherapy, 18 patients had extra-regional lymph node metastasis, 5 had liver metastasis, 8 had macroscopic peritoneal metastasis, and 8 had pancreatic head invasion. Twenty-three (74.2%) of the 31 patients underwent R0 resection. Postoperative morbidity and mortality rates were 16.1 and 0%. During chemotherapy, grade 3/4 toxicities included neutropenia (54.8%), leukopenia (32.3%), and anemia (16.1%). Median progression-free survival and median overall survival (OS) were 42.1 and 56.1 months, respectively. These results were similar for all patients, except those with locally advanced disease alone. In the multivariate analysis for OS, ypN remained an independent negative prognostic factor (p = 0.018).
Conclusion
Surgical resection after DCS chemotherapy for initially unresectable gastric cancer was safe and provided a reasonable R0 resection rate and good mid-term survival.



Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E86.
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27.
Fujitani K. Overview of adjuvant and neoadjuvant therapy for resectable gastric cancer in the East. Dig Surg. 2013;30:119–29.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7.
Bissery MC, Vrignaud P, Lavelle F. Preclinical profile of docetaxel (taxotere): efficacy as a single agent and in combination. Semin Oncol. 1995;22:3–16.
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–57.
Takechi T, Fujioka A, Matsushima E, Fukushima M. Enhancement of the antitumour activity of 5-fluorouracil (5-FU) by inhibiting dihydropyrimidine dehydrogenase activity (DPD) using 5-chloro-2,4-dihydroxypyridine (CDHP) in human tumour cells. Eur J Cancer. 2002;38:1271–7.
Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993;53:4004–9.
Koizumi W, Nakayama N, Tanabe S, Sasaki T, Higuchi K, Nishimura K, et al. A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601). Cancer Chemother Pharmacol. 2012;69:407–13.
Sym SJ, Chang HM, Ryu MH, Lee JL, Kim TW, Yook JH, et al. Neoadjuvant docetaxel, capecitabine and cisplatin (DXP) in patients with unresectable locally advanced or metastatic gastric cancer. Ann Surg Oncol. 2010;17:1024–32.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–85.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 3rd English edition. Gastric Cancer. 2011;14:101–12.
Kurokawa Y, Shibata T, Sasako M, Sano T, Tsuburaya A, Iwasaki Y, et al. Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer. 2014;17:514–21.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Hirakawa M, Sato Y, Ohnuma H, Takayama T, Sagawa T, Nobuoka T, et al. A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother Pharmacol. 2013;71:789–97.
Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303–8.
Oki E, Emi Y, Kusumoto T, Sakaguchi Y, Yamamoto M, Sadanaga N, et al. Phase II Study of Docetaxel and S-1 (DS) as Neoadjuvant Chemotherapy for Clinical Stage III Resectable Gastric Cancer. Ann Surg Oncol 2014;21:2340–6.
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–60.
Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, et al. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer. 2017;20:322–31.
Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96:1015–22.
Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–9.
Han DS, Suh YS, Kong SH, Lee HJ, Im SA, Bang YJ, et al. Outcomes of surgery aiming at curative resection in good responder to induction chemotherapy for gastric cancer with distant metastases. J Surg Oncol. 2013;107:511–6.
Ishigami S, Natsugoe S, Nakajo A, Matsumoto M, Uenosono Y, Arigami T, et al. Salvage gastrectomy following a combination of biweekly paclitaxel and S-1 for stage IV gastric cancer. J Gastrointest Surg. 2008;12:1370–5.
Yabusaki H, Nashimoto A, Matsuki A, Aizawa M. Significance of surgical treatment in multimodal therapy for stage IV highly advanced gastric cancer. Hepatogastroenterology. 2013;60:377–81.
Yano M, Shiozaki H, Inoue M, Tamura S, Doki Y, Yasuda T, et al. Neoadjuvant chemotherapy followed by salvage surgery: effect on survival of patients with primary noncurative gastric cancer. World J Surg. 2002;26:1155–9.
Terashima M. Conversion therapy for gastric cancer: who can make conversion as successful as Goromaru? Gastric Cancer. 2016;19:685–6.
Yoshida K, Yamaguchi K, Okumura N, Osada S, Takahashi T, Tanaka Y, et al. The roles of surgical oncologists in the new era: minimally invasive surgery for early gastric cancer and adjuvant surgery for metastatic gastric cancer. Pathobiology. 2011;78:343–52.
Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19:329–38.
Mansour JC, Tang L, Shah M, Bentrem D, Klimstra DS, Gonen M, et al. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol. 2007;14:3412–8.
Koh YW, Park YS, Ryu MH, Ryoo BY, Park HJ, Yook JH, et al. Postoperative nodal status and diffuse-type histology are independent prognostic factors in resectable advanced gastric carcinomas after preoperative chemotherapy. Am J Surg Pathol. 2013;37:1022–9.
Ojima T, Nakamori M, Nakamura M, Katsuda M, Hayata K, Nakamura Y, et al. Expression of BRCA1, a factor closely associated with relapse-free survival, in patients who underwent neoadjuvant chemotherapy with docetaxel, cisplatin, and fluorouracil for squamous cell carcinoma of the esophagus. Surg Today. 2017;47:65–73.
Wada N, Kurokawa Y, Miyazaki Y, Makino T, Takahashi T, Yamasaki M, et al. The characteristics of the serum carcinoembryonic antigen and carbohydrate antigen 19–9 levels in gastric cancer cases. Surg Today. 2017;47:227–32.
Fields RC, Strong VE, Gonen M, Goodman KA, Rizk NP, Kelsen DP, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer. 2011;104:1840–7.
Cho H, Nakamura J, Asaumi Y, Yabusaki H, Sakon M, Takasu N, et al. Long-term survival outcomes of advanced gastric cancer patients who achieved a pathological complete response with neoadjuvant chemotherapy: a systematic review of the literature. Ann Surg Oncol. 2015;22:787–92.
Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, et al. Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer: early results of the randomized phase II COMPASS trial. Ann Surg Oncol. 2014;21:213–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Mieno, H., Yamashita, K., Hosoda, K. et al. Conversion surgery after combination chemotherapy of docetaxel, cisplatin and S-1 (DCS) for far-advanced gastric cancer. Surg Today 47, 1249–1258 (2017). https://doi.org/10.1007/s00595-017-1512-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1512-z