Abstract
Aims
Diabetic retinopathy (DR) is a vascular complication of diabetes mellitus that causes visual impairment and blindness. Long noncoding RNAs (lncRNAs) have been revealed to be involved in biological processes of several diseases including DR. We designed this study to investigate the specific role of TPTEP1 in DR.
Methods
First, we mimicked diabetic conditions with high glucose (HG) stimulation of human retinal vascular endothelial cells (HRVECs) and measured TPTEP1 expression in HG-stimulated HRVECs using RT-qPCR analysis. Then, CCK-8, Transwell, and Matrigel tube formation assays as well as western blot analysis were performed to reveal the biological functions of TPTEP1 in HG-stimulated HRVECs. Subsequently, bioinformatics analysis, RNA pull down, luciferase reporter and ChIP assays as well as western blot analysis evaluated the relationship of TPTEP1, signal transducer and activator of transcription 3 (STAT3) and vascular endothelial growth factor A (VEGFA) in HG-stimulated HRVECs. Finally, to verify the regulation of the TPTEP1/STAT3/VEGFA axis in HG-stimulated HRVECs, rescue experiments were carried out in HG-stimulated HRVECs.
Results
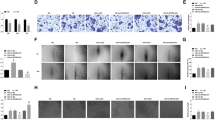
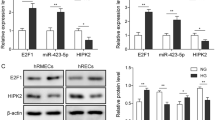
TPTEP1 presented a significant downregulation in HG-stimulated HRVECs. Additionally, TPTEP1 overexpression reduced viability, migration, and angiogenesis in HG-stimulated HRVECs. Moreover, TPTEP1 suppressed phosphorylation and nuclear translocation of STAT3, and thereby downregulated VEGFA mRNA and protein levels. Furthermore, TPTEP1 overexpression-mediated suppression of HG-induced dysfunction in HRVECs was countervailed by STAT3 upregulation or VEGFA upregulation.
Conclusions
TPTEP1 alleviated HG-induced dysfunction in HRVECs via interacting with STAT3 and targeting VEGFA.






Similar content being viewed by others
Change history
09 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00592-022-01979-9
References
Pascolini D, Mariotti SP (2012) Global estimates of visual impairment: 2010. Br J Ophthalmol 96(5):614–618. https://doi.org/10.1136/bjophthalmol-2011-300539
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376(9735):124–136. https://doi.org/10.1016/s0140-6736(09)62124-3
Cunha-Vaz J, Bernardes R, Lobo C (2011) Blood-retinal barrier. Eur J Ophthalmol 21(Suppl 6):S3-9. https://doi.org/10.5301/ejo.2010.6049
Klaassen I, Van Noorden CJ, Schlingemann RO (2013) Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 34:19–48. https://doi.org/10.1016/j.preteyeres.2013.02.001
Erickson KK, Sundstrom JM, Antonetti DA (2007) Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis 10(2):103–117. https://doi.org/10.1007/s10456-007-9067-z
Miyamoto N, de Kozak Y, Jeanny JC, et al. (2007) Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia 50(2):461–470. https://doi.org/10.1007/s00125-006-0539-2
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85(3):845–881. https://doi.org/10.1152/physrev.00021.2004
Cunha-Vaz JG, Travassos A (1984) Breakdown of the blood-retinal barriers and cystoid macular edema. Surv Ophthalmol 28(Suppl):485–492. https://doi.org/10.1016/0039-6257(84)90230-3
Zhu K, Hu X, Chen H, et al. (2019) Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine 49:341–353. https://doi.org/10.1016/j.ebiom.2019.10.004
Yang WZ, Yang J, Xue LP, Xiao LB, Li Y (2017) MiR-126 overexpression inhibits high glucose-induced migration and tube formation of rhesus macaque choroid-retinal endothelial cells by obstructing VEGFA and PIK3R2. J Diabetes Complicat 31(4):653–663. https://doi.org/10.1016/j.jdiacomp.2016.12.004
Mei X, Zhou L, Zhang T, Lu B, Sheng Y, Ji L (2018) Chlorogenic acid attenuates diabetic retinopathy by reducing VEGF expression and inhibiting VEGF-mediated retinal neoangiogenesis. Vasc Pharmacol 101:29–37. https://doi.org/10.1016/j.vph.2017.11.002
Huang Q, Sheibani N (2008) High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol 295(6):C1647-1657. https://doi.org/10.1152/ajpcell.00322.2008
Gao R, Zhu BH, Tang SB, Wang JF, Ren J (2008) Scutellarein inhibits hypoxia- and moderately-high glucose-induced proliferation and VEGF expression in human retinal endothelial cells. Acta Pharmacol Sin 29(6):707–712. https://doi.org/10.1111/j.1745-7254.2008.00797.x
Paraskevopoulou MD, Hatzigeorgiou AG (2016) Analyzing MiRNA-LncRNA interactions. Methods Mol Biol 1402:271–286. https://doi.org/10.1007/978-1-4939-3378-5_21
Michalik KM, You X, Manavski Y, et al. (2014) Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 114(9):1389–1397. https://doi.org/10.1161/circresaha.114.303265
Chinnappan M, Gunewardena S, Chalise P, Dhillon NK (2019) Analysis of lncRNA-miRNA-mRNA interactions in hyper-proliferative human pulmonary arterial smooth muscle cells. Sci Rep 9(1):10533. https://doi.org/10.1038/s41598-019-46981-4
Tang L, Liang Y, Xie H, Yang X, Zheng G (2020) Long non-coding RNAs in cutaneous biology and proliferative skin diseases: advances and perspectives. Cell Prolif 53(1):e12698. https://doi.org/10.1111/cpr.12698
Zhang Z, Salisbury D, Sallam T (2018) Long noncoding RNAs in atherosclerosis: JACC review topic of the week. J Am Coll Cardiol 72(19):2380–2390. https://doi.org/10.1016/j.jacc.2018.08.2161
Zhang X, Zou X, Li Y, Wang Y (2019) Downregulation of lncRNA BANCR participates in the development of retinopathy among diabetic patients. Exp Ther Med 17(5):4132–4138. https://doi.org/10.3892/etm.2019.7444
Luo R, Jin H, Li L, Hu YX, Xiao F (2020) Long noncoding RNA MEG3 inhibits apoptosis of retinal pigment epithelium cells induced by high glucose via the miR-93/Nrf2 axis. Am J Pathol 190(9):1813–1822. https://doi.org/10.1016/j.ajpath.2020.05.008
Thomas AA, Biswas S, Feng B, Chen S, Gonder J, Chakrabarti S (2019) lncRNA H19 prevents endothelial-mesenchymal transition in diabetic retinopathy. Diabetologia 62(3):517–530. https://doi.org/10.1007/s00125-018-4797-6
Ding H, Liu J, Zou R, Cheng P, Su Y (2019) Long non-coding RNA TPTEP1 inhibits hepatocellular carcinoma progression by suppressing STAT3 phosphorylation. J Exp Clin Cancer Res CR 38(1):189. https://doi.org/10.1186/s13046-019-1193-0
Carlevaro-Fita J, Johnson R (2019) Global positioning system: understanding long noncoding RNAs through subcellular localization. Mol Cell 73(5):869–883. https://doi.org/10.1016/j.molcel.2019.02.008
Ding H, Zhang X, Su Y, Jia C, Dai C (2020) GNAS promotes inflammation-related hepatocellular carcinoma progression by promoting STAT3 activation. Cell Mol Biol Lett 25:8. https://doi.org/10.1186/s11658-020-00204-1
Huang Q, Zhong Y, Dong H, et al. (2020) Revisiting signal transducer and activator of transcription 3 (STAT3) as an anticancer target and its inhibitor discovery: where are we and where should we go? Eur J Med Chem 187:111922. https://doi.org/10.1016/j.ejmech.2019.111922
Huang J, Tang L, Zhao Y, Ding W (2019) TRIM11 promotes tumor angiogenesis via activation of STAT3/VEGFA signaling in lung adenocarcinoma. Am J Cancer Res 9(9):2019–2027
Zhao J, Du P, Cui P, et al. (2018) LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene 37(30):4094–4109. https://doi.org/10.1038/s41388-018-0250-z
Wu X, Xiao Y, Zhou Y, Zhou Z, Yan W (2019) LncRNA FOXP4-AS1 is activated by PAX5 and promotes the growth of prostate cancer by sequestering miR-3184-5p to upregulate FOXP4. Cell Death Dis 10(7):472. https://doi.org/10.1038/s41419-019-1699-6
Nagaraju GP, Park W, Wen J, et al. (2013) Antiangiogenic effects of ganetespib in colorectal cancer mediated through inhibition of HIF-1α and STAT-3. Angiogenesis 16(4):903–917. https://doi.org/10.1007/s10456-013-9364-7
Dong Z, Santeford A, Ban N, et al. (2019) FGF2-induced STAT3 activation regulates pathologic neovascularization. Exp Eye Res 187:107775. https://doi.org/10.1016/j.exer.2019.107775
Sui H, Zhao J, Zhou L, et al. (2017) Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett 403:86–97. https://doi.org/10.1016/j.canlet.2017.05.013
Stitt AW, Curtis TM, Chen M, et al. (2016) The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 51:156–186. https://doi.org/10.1016/j.preteyeres.2015.08.001
Elshaer SL, Lemtalsi T, El-Remessy AB (2018) High glucose-mediated tyrosine nitration of PI3-Kinase: a molecular switch of survival and apoptosis in endothelial cells. Antioxidants. https://doi.org/10.3390/antiox7040047
Terlizzi V, Kolibabka M, Burgess JK, Hammes HP, Harmsen MC (2018) The pericytic phenotype of adipose tissue-derived stromal cells is promoted by NOTCH2. Stem Cells 36(2):240–251. https://doi.org/10.1002/stem.2726
Fu X, Ou B (2020) miR-152/LIN28B axis modulates high-glucose-induced angiogenesis in human retinal endothelial cells via VEGF signaling. J Cell Biochem 121(2):954–962. https://doi.org/10.1002/jcb.28978
Cao F, Wang Z, Feng Y, et al. (2020) lncRNA TPTEP1 competitively sponges miR-328-5p to inhibit the proliferation of non-small cell lung cancer cells. Oncol Rep 43(5):1606–1618. https://doi.org/10.3892/or.2020.7522
Zhu M, Liu X, Wang Y, et al. (2018) YAP via interacting with STAT3 regulates VEGF-induced angiogenesis in human retinal microvascular endothelial cells. Exp Cell Res 373(1–2):155–163. https://doi.org/10.1016/j.yexcr.2018.10.007
Chen LY, Wang X, Qu XL, et al. (2019) Activation of the STAT3/microRNA-21 pathway participates in angiotensin II-induced angiogenesis. J Cell Physiol 234(11):19640–19654. https://doi.org/10.1002/jcp.28564
Zheng XJ, Liu Y, Zhang WC, et al. (2019) Mineralocorticoid receptor negatively regulates angiogenesis through repression of STAT3 activity in endothelial cells. J Pathol 248(4):438–451. https://doi.org/10.1002/path.5269
Taimeh Z, Loughran J, Birks EJ, Bolli R (2013) Vascular endothelial growth factor in heart failure. Nat Rev Cardiol 10(9):519–530. https://doi.org/10.1038/nrcardio.2013.94
Apte RS, Chen DS, Ferrara N (2019) VEGF in signaling and disease: beyond discovery and development. Cell 176(6):1248–1264. https://doi.org/10.1016/j.cell.2019.01.021
Melincovici CS, Boşca AB, Şuşman S, et al. (2018) Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol Rev Roum de Morphol et Embryol 59(2):455–467
Xie J, Zhang D, Ling Y, et al. (2019) Substrate elasticity regulates vascular endothelial growth factor A (VEGFA) expression in adipose-derived stromal cells: implications for potential angiogenesis. Colloids Surf B Biointerfaces 175:576–585. https://doi.org/10.1016/j.colsurfb.2018.08.035
Acknowledgements
Thank you for all laboratory members involved in this study.
Funding
This work supported by Clinical Specialized Discipline of Health System of Putuo District in Shanghai (No. 2020TSZK01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there exists no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Ethical approval
Not applicable.
Additional information
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Correct version of figures tube formation assays (Figure 2D&6D) and Transwell assays (Figure 2C&6C) updated.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, X., Lu, Y. & Lei, T. TPTEP1 suppresses high glucose-induced dysfunction in retinal vascular endothelial cells by interacting with STAT3 and targeting VEGFA. Acta Diabetol 58, 759–769 (2021). https://doi.org/10.1007/s00592-020-01663-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01663-w