Abstract
Aims
To investigate whether intravitreal conbercept injection affects contralateral untreated eyes in bilateral diabetic macular edema (DME) patients.
Methods
In this retrospective study, 15 patients (30 eyes) with type 2 diabetes were followed after bilateral DME diagnosis in the Department of Ophthalmology, Peking Union Medical College Hospital from 2015 to 2018. Patients underwent examinations including best corrected visual acuity (BCVA), slit-lamp microscopy, indirect ophthalmoscope, color fundus photography, fundus fluorescein angiography, optical coherence tomography, and glycated hemoglobin (HbA1c). Each patient received conbercept (0.5 mg) intravitreally in the severe eye. Nonparametric Wilcoxon signed-rank tests and Pearson’s correlation coefficient were used to assess changes in BCVA and central retinal thickness (CRT) and relations between BCVA changes in treated and untreated eyes, respectively.
Results
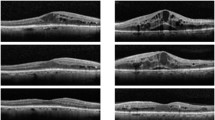
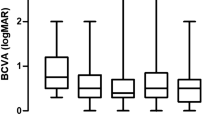
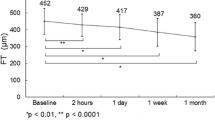
The mean follow-up time was 10.60 ± 2.29 months, and the mean injection number of 15 treated eyes was 9.13 ± 0.68. HbA1c remained below 10% during treatment with no significant changes between the initial and final visits (7.81 ± 1.17 vs 7.62 ± 1.19%) (P = 0.576). In untreated eyes, CRT significantly decreased from the initial to final visits (368.93 ± 125.45 vs 306.27 ± 89.70 μm) (P = 0.028). In untreated eyes, BCVA showed no significant difference between the initial and final visits (0.38 ± 0.30 vs 0.40 ± 0.30 logMAR) (P = 0.937), but BCVA changes in treated and untreated eyes were positively correlated (r = 0.527, P = 0.044).
Conclusions
Intravitreal conbercept injection results in decreased CRT and increased BCVA in untreated eyes, which is consistent with the changes in treated eyes for patients with bilateral DME.



Similar content being viewed by others
References
Ting DS, Cheung GC, Wong TY (2016) Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol 44:260–277
Korobelnik JF, Do DV, Schmidt-Erfurth U et al (2014) Intravitreal aflibercept for diabetic macular edema. Ophthalmology 121:2247–2254
Brown DM, Schmidt-Erfurth U, Do DV et al (2015) Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 122:2044–2052
Staurenghi G, Feltgen N, Arnold JJ et al (2018) Impact of baseline diabetic retinopathy severity scale scores on visual outcomes in the VIVID-DME and VISTA-DME studies. Br J Ophthalmol 102:954–958
Laiginhas R, Silva MI, Falcão MS (2018) Reply to the letter to the editor: aflibercept in diabetic macular edema refractory to previous bevacizumab: outcomes and predictors of success. Graefes Arch Clin Exp Ophthalmol 256:1355–1356
Herbaut A, Fajnkuchen F, Qu-Knafo L et al (2017) Switching to aflibercept in diabetic macular edema not responding to ranibizumab and / or intravitreal dexamethasone implant. J Ophthalmol 2017:8035013
Li F, Zhang Le, Wang Y et al (2018) One-year outcome of combercept therapy for diabetic macular edema. Curr Eye Res 43:218–223
Funatsu H, Yamashita H, Nakamura S et al (2006) Vitreous levels of pigment epithelial-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology 113:294–301
Wells JA, Glassman AR, Ayala AR et al (2015) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372:1193–1203
Xu X, Lu Y (2018) Focusing on the diagnosis and treatment of diabetic macular edema. Chin J Ocul Fundus Dis 34:313–316
Bakbak B, Ozturk BT, Gonul S et al (2013) Comparison of the effect of unilateral intravitreal bevacizumab and ranibizumab injection on diabetic macular edema of the fellow eye. J Ocul Pharmacol Ther 29:728–732
Hanhart J, Tiosano L, Averbukn E et al (2014) Fellow eye effect of unilateral intavitreal bevacizumab injection in eyes with diabetic macular edema. Eye 28:646–653
Acharya NR, Sittivarakul W, Qian Y et al (2011) Bilateral effect of unilateral ranibizumab in patients with patients with uveitis-related macular edema. Retina 31:1871–1876
Zq Wu, Sadda SR (2018) Effects on the contralateral eye after intravitreal bevacizumab and ranibizumab injections: a case report. Ann Acad Med Singap 37:591–593
Avery RL, Castellarin AA, Steinleetal NC et al (2014) Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 98:1636–1641
Huang CY, Lien R, Wangetal NK et al (2018) Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 256:479–487
Bakri SJ, Snyder MR, Reid JM et al (2007) Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 114:855–859
Bakri SJ, Snyder MR, Reid JM et al (2007) Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 114:2179–2182
Dias CS, Mitra AK (2000) Vitreal elimination kinetics of large molecular weight FITC-labeled dextrans in albino rabbits using a novel microsampling technique. J Pharm Sci 89:572–578
Montoya RV, Guerra JF, Burgos O et al (2009) The effect of unilateral intravitreal bevacizumab (Avastin), in the treatment of diffuse bilateral diabetic macular edema a polot study. Retina 29:20–26
Meyer CH, Krohne TU, Holz FG (2012) Concentrations of unbound bevacizumab in the aqueous of untreated fellow eyes after single intravitreal injection in humans. Acta Ophthalmol 90:68–70
Brown DM, Nguyen QD, Marcus DM et al (2013) Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 120:2013–2022
Heier JS, Korobelnik JF, Brown DM et al (2016) Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology 123:2376–2385
Diabetic Retinopathy Clinical Retinopathy Network (2018) Protocol T: a comparative effectiveness study of intravitreal aflibercept, bevacizumab and ranibizumab for diabetic macular edema [EB/OL]. https://drcrnet.jaeb.org. 2018-10-18/2019-4-30. Accessed 30 Apr 2019
Diabetic Retinopathy Clinical Retinopathy Network (2013) Protocol I: intravitreal ranibizumab or triamcinolone acetonide in combination with laser photocoagulation for diabetic macular edema [EB/OL]. https://drcrnet.jaeb.org. 2013-12-31/2019-4-30. Accessed 30 Apr 2019
Hammes HP, Welp R, Kempe HP et al (2015) Risk factors for retinopathy and DME in type 2 diabetes-results from the German/Austrian DPV Database. PLoS ONE 10:e0132492
Yau JW, Rogers SL, Kawasaki R (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564
Author information
Authors and Affiliations
Contributions
YD and ZL carried out the entire procedure including the collection of medical records, statistical analysis, drafting the manuscript, and manuscript revision. JY conceived of the study, coordinated and participated in the entire process of drafting, and revised the manuscript. LL contributed to manuscript revision. All authors read and approved the final manuscript. BL and RY contribute to the collection of medical records and statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
All authors have contributed significantly and agree with the content of the manuscript. All authors have no relevant financial relationships to disclose.
Ethical approval
The study was approved by the hospital’s institutional review board and was conducted in accordance with the tenets of the Declaration of Helsinki. The reference number for the ethics approval is NO. S-K848.
Informed consent
The authors ensured that patients’ privacy is well protected in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the topical collection Eye Complications of Diabetes, managed by Giuseppe Querques.
Rights and permissions
About this article
Cite this article
Di, Y., Li, Z., Ye, J. et al. The fellow eye effect of unilateral intravitreal conbercept injections in eyes with diabetic macular edema. Acta Diabetol 57, 1001–1007 (2020). https://doi.org/10.1007/s00592-020-01511-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-020-01511-x