Abstract
Aims
Islet transplantation is an effective therapeutic option for type 1 diabetes. Although maintenance immunosuppression therapy is required to prevent allogeneic rejection and recurrence of autoimmunity, long-term allograft survival has not yet been achieved partly because of its adverse effects. The induction of donor-specific immunotolerance is a promising approach for long-term allograft survival without maintenance immunosuppression therapy. We previously reported that combination therapy using a liposomal ligand for invariant natural killer T cells, RGI-2001, and anti-CD154 antibody established mixed hematopoietic chimerism for the induction of donor-specific immunotolerance. This study investigated whether the protocol could promote islet allograft acceptance in experimental diabetes.
Methods
Streptozotocin-induced diabetic BALB/c mice were transplanted with bone marrow cells from C57BL/6 donors and received combination therapy of RGI-2001 and anti-CD154 antibody after 3-Gy total body irradiation. 3 Weeks after bone marrow transplantation, islets isolated from C57BL/6 donors were transplanted under the kidney capsule.
Results
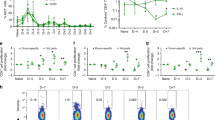
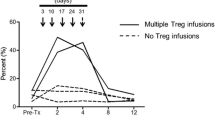
Mixed chimerism was established in diabetic mice receiving the tolerance induction protocol. After islet transplantation, blood glucose levels improved and normoglycemia persisted for over 100 days. Hyperglycemia recurred after islet grafts were removed. Histopathological examinations showed insulin-positive staining and absence of cellular infiltration in the islet grafts. T cells of recipients showed donor-specific hyporesponsiveness, and anti-donor antibodies were not detected.
Conclusions
The tolerance induction protocol with combination therapy of RGI-2001 and anti-CD154 antibody promoted islet allograft acceptance in a mouse diabetic model. This protocol may be clinically applied to islet transplantation for type 1 diabetes mellitus.





Similar content being viewed by others
References
National Clinical Guideline Centre (UK, 2015) (2015) Type 1 diabetes in adults: diagnosis and management. NICE Guideline No. 17 [online guideline]. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0079032/. Accessed 6 June 2018
Robertson RP (2015) Islet transplantation for type 1 diabetes what have we learned from alloislet and autoislet successes? Diabetes Care 38:1030–1035
McCall M, Shapiro AM (2012) Update on islet transplantation. Cold Spring Harb Perspect Med 2012 2:a007823
Barton FB, Rickels MR, Alejandro R et al (2012) Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35:1436–1445
Brooks AM, Walker N, Aldibbiat A et al (2013) Attainment of metabolic goals in the integrated UK islet transplant program with locally isolated and transported preparations. Am J Transpl 13:3236–3243
Bensellam M, Laybutt DR, Jonas JC (2012) The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol Cell Endocrinol 364:1–27
Talchai C, Lin HV, Kitamura T, Accili D (2009) Genetic and biochemical pathways of β-cell failure in type 2 diabetes. Diabetes Obes Metab 11:38–45
Wallace M, Whelan H, Brennan L (2013) Metabolomic analysis of pancreatic beta cells following exposure to high glucose. Biochim Biophys Acta 1830:2583–2590
Jonas JC, Bensellam M, Duprez J, Elouil H, Guiot Y, Pascal SM (2009) Glucose regulation of islet stress responses and β cell failure in type 2 diabetes. Diabetes Obes Metab 11:65–81
Kikawa K, Sakano D, Shiraki N et al (2014) Beneficial effect of insulin treatment on islet transplantation outcomes in Akita mice. PLoS One 9(4):e95451
Sweet IR, Gilbert M, Scott S et al (2008) Glucose-stimulated increment in oxygen consumption rate as a standardized test of human islet quality. Am J Transpl 8:183–192
Matschinsky FM (1996) Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 45:223–241
Mathew JM, Voss JH, McEwen ST et al (2018) Generation and characterization of alloantigen-specific regulatory T cells for clinical transplant tolerance. Sci Rep 8:1136
Shapiro AM (2013) Immune antibody monitoring predicts outcome in islet transplantation. Diabetes 62:1377–1378
Jianming T, Shunliang Y, Jinquan C et al (2008) Simultaneous islet-kidney transplantation in seven patients of Type 1 diabetes with end-stage renal disease using a glucocorticoid-free immunosuppressive regimen with alemtuzumab induction. Diabetes 57:2666–2671
Danielle JB, Marc W, Carmen W et al (2014) Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngenic mouse model. Diabetologia 57:522–531
Hirai T, Ishii Y, Ikemiyagi M et al (2014) A novel approach inducing transplant tolerance by activated invariant natural killer T cells with costimulatory blockade. Am J Transpl 14:554–567
Hirai T, Ishii R, Miyairi S et al (2016) Clonal deletion established via invariant NKT cell activation and costimulatory blockade requires in vivo expansion of regulatory T cells. Am J Transpl 16:426–439
Miyairi S, Hirai T, Ishii R et al (2017) Donor bone marrow cells are essential for iNKT cell-mediated Foxp3+ Treg cell expansion in a murine model of transplantation tolerance. Eur J Immunol 47:734–742
Ishii R, Hirai T, Miyairi S et al (2018) iNKT cell activation plus T-cell transfer establishes complete chimerism in a murine sublethal bone marrow transplant model. Am J Transpl 18:328–340
Chen YB, Efebera YA, Johnston L et al (2017) Increased Foxp3(+)Helios(+) regulatory T cells and decreased acute graft-versus-host disease after allogeneic bone marrow transplantation in patients receiving sirolimus and RGI-2001, an activator of invariant natural killer T cells. Biol Blood Marrow Transpl 23:625–634
Duramad O, Laysang A, Li J, Ishii Y, Namikawa R (2011) Pharmacologic expansion of donor-derived, naturally occurring CD4(+)Foxp3(+) regulatory T cells reduces acute graft-versus-host disease lethality without abrogating the graft-versus-leukemia effect in murine models. Biol Blood Marrow Transpl 17:1154–1168
Lorenzo P, Matthew JE, Paola M et al (2013) Alloantibody and autoantibody monitoring predicts islet transplantation outcome in human type 1 diabetes. Diabetes 62:1656–1664
Brooks AMS, Carter V, Liew A et al (2015) De novo donor-specific HLA antibodies are associated with rapid loss of graft function following islet transplantation in type 1 diabetes. Am J Transpl 15:3239–3246
Li H, Inveradi L, Molano RD, Pileggi A, Ricordi C (2003) Nonlethal conditioning for the induction of allogeneic chimerism and tolerance to islet allografts. Transplantation 75:966–970
Luo B, Nanji SA, Schur CD, Pawlick RL, Anderson CC, Shapiro AM (2005) Robust tolerance to fully allogeneic islet transplants achieved by chimerism with minimal conditioning. Transplantation 80:370–377
Liang Y, Huang T, Zhang C et al (2005) Donor CD8+ T cells facilitate induction of chimerism and tolerance without GVHD in autoimmune NOD mice conditioned with anti-CD3 mAb. Blood 105:2180–2188
Racine J, Wang M, Zhang C et al (2011) Induction of mixed chimerism with MHC-mismatched but not matched bone marrow transplants results in thymic deletion of host-type autoreactive T-cells in NOD mice. Diabetes 60:555–564
Wang M, Racine J, Zhang M et al (2014) MHC-mismatched chimerism is required for induction of transplantation tolerance in autoimmune nonobese diabetic recipients. J Immunol 193:2005–2015
William FNC, Razavy H, Luo B et al (2008) Development of either split tolerance or robust tolerance along with humoral tolerance to donor and third-party alloantigens in nonmyeloablative mixed chimeras. J Immunol 180:5177–5186
Al-Adra DP, Pawlick R, Shapiro AMJ, Anderson CC (2012) Targeting cells causing split tolerance allows fully allogeneic islet survival with minimal conditioning in NOD mixed chimeras. Am J Transpl 12:3235–3245
Beilhak GF, Scheffold YC, Weissman IL et al (2003) Purified allogeneic hematopoietic stem cell transplantation blocks diabetes pathogenesis in NOD mice. Diabetes 52:59–68
Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M (2004) Mixed hematopoietic chimerism allow cure of autoimmune diabetes through allogeneic tolerance and reversal of autoimmunity. Diabetes 53:376–383
Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB (2000) Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 6:114
Kim SC, Wakwe W, Higginbotham LB et al (2017) Fc-Silent anti-CD154 domain antibody effectively prevents nonhuman primate renal allograft rejection. Am J Transpl 17:1182–1192
Acknowledgements
During this research, we used instruments from the Medical Research Institute (MRI), Tokyo Women’s Medical University. The authors would like to thank Hideki Nakayama for the staining of pathological samples. This work was supported by JSPS KAKENHI Grant number JP15K19864.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Ishii is the executive chairman of REGiMMUNE Corp. The other authors declare that they have no conflict of interest.
Human and animal right disclosure
Animal studies were approved by the Tokyo Women’s Medical University internal committee on the use and care of laboratory animals approved all experiments (Reference ID: AE16-105-2). All animals received human care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23, revised 1985) as well as the current version of the Japanese law “Act on Welfare and Management of Animals” (revised 2013).
Informed consent
For this type of study formal informed consent is not required.
Additional information
Managed by Massimo Porta.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kanzawa, T., Hirai, T., Fukuda, H. et al. Combination therapy of an iNKT cell ligand and CD40–CD154 blockade establishes islet allograft acceptance in nonmyeloablative bone marrow transplant recipients. Acta Diabetol 56, 541–550 (2019). https://doi.org/10.1007/s00592-019-01289-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01289-7