Abstract
Aims
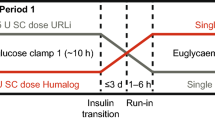
The objective of the study was to compare the pharmacokinetic (PK) and pharmacodynamic (PD) properties of an insulin glargine formulation, Glaritus® (test) with the innovator’s formulation Lantus® (reference) using the euglycemic clamp technique in a single-dose, double-blind, randomized, two sequences, four-period replicate crossover study in healthy volunteers (n = 40).
Methods
Subjects received subcutaneous administration of the insulin glargine (0.4 IU/kg) formulation at two occasions for test and reference and a 20% glucose solution was infused at variable rate to maintain euglycemia for 24 h.
Results
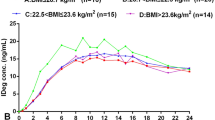
Both PK [area under the plasma concentration time curve (AUC0–24 h) and maximum insulin concentration (Cmax)] and PD endpoints [area under glucose infusion rate time curve (AUCGIR0–24) and maximum glucose infusion rate (GIRmax)] demonstrated bioequivalence of Glaritus to Lantus with the 90% confidence interval of geometric mean ratio of test to reference entirely contained within 0.80–1.25. Both formulations showed equivalent geometric least-square mean LSM value (0.08 nmol/L) for Cmax. The geometric LSM AUC0–24 h value for Glaritus® (1.09 h nmol/L) was comparable to Lantus (1.05 h nmol/L). Median Tmax values were also identical (12 h for both), and median t1/2 values were also equal (18 h for both). For GIRTmax, the difference between the means for the two was not statistically significant. No AEs related to study formulations were reported, and both products were well tolerated.
Conclusions
The test product (Glaritus) was found to be bioequivalent to the reference product (Lantus).
Clinical trial registration number
CTRI/2015/06/005890; http://www.ctri.nic.in/.




Similar content being viewed by others
Change history
17 November 2020
Authors would like to correct the error in table��2 of the online published article.
References
Owens DR, Landgraf W, Schmidt A et al (2012) The emergence of biosimilar insulin preparations—a cause for concern? Diabetes Technol Ther 14:989–996. https://doi.org/10.1089/dia.2012.0105
Lepore M, Pampanelli S, Fanelli C et al (2000) Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 49:2142–2148
Scholtz HE, Pretorius SG, Wessels DH, Becker RHA (2005) Pharmacokinetic and glucodynamic variability: assessment of insulin glargine, NPH insulin and insulin ultralente in healthy volunteers using a euglycaemic clamp technique. Diabetologia 48:1988–1995. https://doi.org/10.1007/s00125-005-1916-y
Rotenstein LS, Ran N, Shivers JP et al (2012) Opportunities and challenges for biosimilars: what’s on the horizon in the global insulin market? Clin Diabetes 30:138–150
Ratner RE, Hirsch IB, Neifing JL et al (2000) Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. US Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care 23:639–643
Ashwell SG, Amiel SA, Bilous RW (2006) Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross-over trial in people with Type 1 diabetes. Diabet Med 23:285–292
Barnett AH (2006) Insulin glargine in the treatment of type 1 and type 2 diabetes. Vasc Health Risk Manag 2:59
Rys P, Wojciechowski P, Rogoz-Sitek A et al (2015) Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol 52:649–662. https://doi.org/10.1007/s00592-014-0698-4
Pérez-Maraver M, Caballero-Corchuelo J, Boltana A et al (2013) Comparison of human insulin and insulin analogues on hypoglycaemia and metabolic variability in type 1 diabetes using standardized measurements (HYPO score and Lability Index). Acta Diabetol 50:529–535. https://doi.org/10.1007/s00592-011-0320-y
Cigrovski Berkovic M, Petrovski G, Grulovic N (2016) Effectiveness of insulin glargine in type 2 diabetes mellitus patients failing glycaemic control with premixed insulin: adriatic countries data meta-analysis. Acta Diabetol 53:709–715. https://doi.org/10.1007/s00592-016-0861-1
Hasani-Ranjbar S, Fazlollahi MR, Mehri A, Larijani B (2012) Allergy to human insulin and specific immunotherapy with glargine; case report with review of literature. Acta Diabetol 49:69–73. https://doi.org/10.1007/s00592-011-0254-4
Akinci B, Yener S, Bayraktar F, Yesil S (2010) Allergic reactions to human insulin: a review of current knowledge and treatment options. Endocrine 37:33–39. https://doi.org/10.1007/s12020-009-9256-1
Heinzerling L, Raile K, Rochlitz H et al (2008) Insulin allergy: clinical manifestations and management strategies: insulin allergy. Allergy 63:148–155. https://doi.org/10.1111/j.1398-9995.2007.01567.x
Puppalwar G, Sawant S, Silgiri B et al (2017) Evaluation of Safety and Efficacy of Glaritus® versus Lantus® in Combination with Insulin Lispro among Adults with Type 1 Diabetes Mellitus-Phase IV Study. Open J Endocr Metab Dis 07:111–125. https://doi.org/10.4236/ojemd.2017.74011
Bhuiyan PS, Rege NN (2001) ICH harmonised tripartite guideline: guideline for good clinical practice. J Postgrad Med 47:45–50
GUIDELINE FOR GOOD CLINICAL PRACTICE - E6_R1_Guideline.pdf. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 3 Aug 2017
Linnebjerg H, Lam ECQ, Seger ME et al (2015) Comparison of the pharmacokinetics and pharmacodynamics of LY2963016 insulin glargine and EU- and US-approved versions of Lantus insulin glargine in healthy subjects: three randomized euglycemic clamp studies. Diabetes Care 38:2226–2233. https://doi.org/10.2337/dc14-2623
Heise T, Nosek L, Rønn BB et al (2004) Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 53:1614–1620
Lucidi P, Porcellati F, Candeloro P et al (2014) Glargine metabolism over 24 h following its subcutaneous injection in patients with type 2 diabetes mellitus: a dose–response study. Nutr Metab Cardiovasc Dis 24:709–716. https://doi.org/10.1016/j.numecd.2014.02.008
Requirements and guidelines for permission to import and/or manufacture of new drugs for sale or to undertake clinical trials. In: Cent. Drugs Stand. Control Organ. Website New Delhi India. http://www.jli.edu.in/blog/wp-content/uploads/2017/01/Drugs-and-Cosmetics-Act-and-Rules2016.pdf. Accessed 17 Aug 2017
Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues - WC500184161.pdf. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184161.pdf. Accessed 3 Aug 2017
Bequette BW (2009) Glucose clamp algorithms and insulin time-action profiles. J Diabetes Sci Technol 3:1005–1013. https://doi.org/10.1177/193229680900300503
Acknowledgements
The authors also acknowledge Veeda Clinical Research for conduct of the study, Manipal Acunova for analysis of PK samples and Devdutt Pathare for monitoring the study.
Author information
Authors and Affiliations
Contributions
Eliford Ngaimisi contributed to data analysis and manuscript writing. Mathangi Gopalakrishnan contributed to data analysis and manuscript writing. Joga Gobburu contributed to manuscript writing. Prasanna Kumar analyzed and interpreted the data. Ashima Bhatia contributed to the study methodology and procedures and data interpretation, Shraddha Tawade, Mushtaque Mastim, Sridhar Yeshamaina and Manish Shah were involved in study design, data collection, data analysis and interpretation. Maharaja Sahib and Dipak Thakur contributed to the development of bioanalysis method of Glargine. All the authors approved the final draft of the manuscript after critical review and revision.
Corresponding author
Ethics declarations
Conflict of interest
Prasanna Kumar K M is an advisory board member of Wockhardt, Sanofi, Biocon, Novo- Nordisk and Eli Lilly and has conducted clinical research as PI for the insulin analogs for these companies. Shraddha Tawade, Mushtaque Mastim, Manish Shah, Sridhar Yeshamaina, Maharaja Sahib, Dipak Thakur and Ashima Bhatia are employees of Wockhardt Ltd. Eliford Ngaimisi, Mathangi Gopalakrishnan and Joga Gobburu have no conflict of interest to declare.
Ethical approval
The study was conducted in accordance with the declaration of Helsinki and as per the guidelines formulated by the ICH GCP and the Indian Council of Medical Research (ICMR) for biomedical research on human subjects.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhatia, A., Tawade, S., Mastim, M. et al. Comparative evaluation of pharmacokinetics and pharmacodynamics of insulin glargine (Glaritus®) and Lantus® in healthy subjects: a double-blind, randomized clamp study. Acta Diabetol 55, 461–468 (2018). https://doi.org/10.1007/s00592-018-1113-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1113-3