Abstract
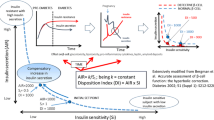
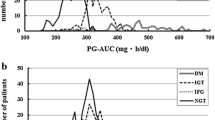
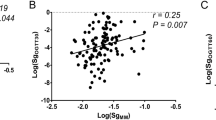
Aim of this study was to formulate an index for glucose effectiveness (Sg), SgIo, based on 3-point (0, 30 and 120 min) 75 g oral glucose tolerance test (OGTT). The equation for SgIO was developed in the Chikuma cohort (n = 502). Firstly, post-loading plasma glucose without insulin action and Sg (PPG-without insulin and Sg) was calculated as follows: fasting plasma glucose (mg/dl) + [0.75 × 75,000]/[0.19 × BW(kg) × 10]. Secondly, ‘PPG-without insulin/with Sg’ was obtained from inverse correlation between log10DIO and 2-h post-glucose plasma glucose at OGTT (2hPG) in each glucose tolerance category: DIO denotes oral disposition index, a product of the Matsuda Index and δIRI0–30/δPG0–30. Thirdly, expected 2hPG (2hPGE) of a given subject was obtained from the regression, and the ratio of 2hPG to 2hPGE (2hPG/2hPGE) was determined as an adjustment factor. Lastly, SgIO ([mg/dl]/min) was calculated as \( \frac{{[{\text{PPG}} \hbox{-} {\text{without}}\;{\text{insulin}}\;{\text{and}}\;{\text{Sg}}] - [{\text{PPG}} \hbox{-} {\text{without}}\;{\text{insulin}}/{\text{with}}\;{\text{Sg}}] \times [(2{\text{hPG}})/(2{\text{hPG}}_{\text{E}})]}}{120} \). SgIO was validated against Sg obtained by frequently sampled intravenous glucose tolerance test in the Jichi cohort (n = 205). Also, the accuracy of prediction of Sg by SgIo was tested by the Bland–Altman plot. SgIO was 3.61 ± 0.73, 3.17 ± 0.74 and 2.15 ± 0.60 in subjects with normal glucose tolerance (NGT), non-diabetic hyperglycemia and diabetes, respectively, in the Chikuma cohort. In the Jichi cohort, SgIO was significantly correlated with Sg in the entire group (r = 0.322, P < 0.001) and in subjects with NGT (r = 0.286, P < 0.001), and SgIo accurately predicted Sg. In conclusion, SgIO could be a simple, quantitative index for Sg.



Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- NGT:
-
Normal glucose tolerance
- NDH:
-
Non-diabetic hyperglycemia
- IFG:
-
Impaired fasting glucose
- IGT:
-
Impaired glucose tolerance
- DM:
-
Diabetes mellitus
- T2DM:
-
Type 2 diabetes
- FSIVGTT:
-
Frequently sampled intravenous glucose tolerance test
- OGTT:
-
Oral glucose tolerance test
- IRI:
-
Immunoreactive insulin
- PPG:
-
Post-loading plasma glucose
- FPG:
-
Fasting plasma glucose
- 2hPG:
-
2-h post-glucose PG
- 2hPGE :
-
Expected 2hPG
- Sg:
-
Glucose effectiveness
- SgIO :
-
Oral Sg index
- SgIVG :
-
Sg determined by FSIVGTT or the clamp method
- SI:
-
Insulin sensitivity index
- DI:
-
Disposition index
- DIO :
-
Oral disposition index
- ISIMatsuda :
-
Insulin sensitivity determined by Matsuda Index
- SMA:
-
Standardized major axis
- SD:
-
Standard deviation
References
Bergman RN, Finegood DT, Ader M (1985) Assessment of insulin sensitivity in vivo. Endocr Rev 6:45–86
Welch S, Gebhart SS, Bergman RN, Phillips LS (1990) Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 71:1508–1518
Taniguchi A, Nakai Y, Fukushima M, Kawamura H, Imura H, Nagata I et al (1992) Pathogenic factors responsible for glucose intolerance in patients with NIDDM. Diabetes 41:1540–1546
Taniguchi A, Nakai Y, Fukushima M, Imura H, Kawamura H, Nagata I et al (1994) Insulin sensitivity, insulin secretion, and glucose effectiveness in subjects with impaired glucose tolerance: a minimal model analysis. Metabolism 43:714–718
Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN (1996) Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 19:1018–1030
Basu A, Caumo A, Bettini F, Gelisio A, Alzaid A, Cobelli C et al (1997) Impaired basal glucose effectiveness in NIDDM: contribution of defects in glucose disappearance and production, measured using an optimized minimal model independent protocol. Diabetes 46:421–432
Nagasaka S, Tokuyama K, Kusaka I, Hayashi H, Rokkaku K, Nakamura T et al (1999) Endogenous glucose production and glucose effectiveness in type 2 diabetic subjects derived from stable-labeled minimal model approach. Diabetes 48:1054–1060
Taniguchi A, Fukushima M, Sakai M, Nagata I, Doi K, Nagasaka S et al (2000) Insulin secretion, insulin sensitivity, and glucose effectiveness in nonobese individuals with varying degrees of glucose tolerance. Diabetes Care 23:127–128
Tokuyama Y, Sakurai K, Yagui K, Hashimoto N, Saito Y, Kanatsuka A (2001) Pathophysiologic phenotypes of Japanese subjects with varying degrees of glucose tolerance: using the combination of C-peptide secretion rate and minimal model analysis. Metabolism 50:812–818
Tonelli J, Kishore P, Lee DE, Hawkins M (2005) The regulation of glucose effectiveness: how glucose modulates its own production. Curr Opin Clin Nutr Metab Care 8:450–456
Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR (1992) Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340:925–929
Osei K, Rhinesmith S, Gaillard T, Schuster D (2004) Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care 27:1439–1446
Lorenzo C, Wagenknecht LE, Karter AJ, Hanley AJ, Rewers MJ, Haffner SM (2011) Cross-sectional and longitudinal changes of glucose effectiveness in relation to glucose tolerance: the insulin resistance atherosclerosis study. Diabetes Care 34:1959–1964
Sakamaki H, Yamasaki H, Matsumoto K, Izumino K, Kondo H, Sera Y et al (1998) No deterioration in insulin sensitivity, but impairment of both pancreatic beta-cell function and glucose sensitivity, in Japanese women with former gestational diabetes mellitus. Diabet Med 15:1039–1044
Nishida Y, Tokuyama K, Nagasaka S, Higaki Y, Shirai Y, Kiyonaga A et al (2004) Effect of moderate exercise training on peripheral glucose effectiveness, insulin sensitivity, and endogenous glucose production in healthy humans estimated by a two-compartment-labeled minimal model. Diabetes 53:315–320
Hayashi Y, Nagasaka S, Takahashi N, Kusaka I, Ishibashi S, Numao S et al (2005) A single bout of exercise at higher intensity enhances glucose effectiveness in sedentary men. J Clin Endocrinol Metab 90:4035–4040
Page R, Boolell M, Kalfas A, Sawyer S, Pestell R, Ward G et al (1991) Insulin secretion, insulin sensitivity and glucose-mediated glucose disposal in Cushing’s disease: a minimal model analysis. Clin Endocrinol 35:509–517
Matsumoto K, Yamasaki H, Akazawa S, Sakamaki H, Ishibashi M, Abiru N et al (1996) High-dose but not low-dose dexamethasone impairs glucose tolerance by inducing compensatory failure of pancreatic beta-cells in normal men. J Clin Endocrinol Metab 81:2621–2626
Nielsen MF, Caumo A, Chandramouli V, Schumann WC, Cobelli C, Landau BR et al (2004) Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercortisolemia in healthy subjects. Am J Physiol Endocrinol Metab 286:E102–E110
Doi K, Taniguchi A, Nakai Y, Kawamura H, Higaki Y, Yokoi H et al (1997) Decreased glucose effectiveness but not insulin resistance in glucose-tolerant offspring of Japanese non-insulin-dependent diabetic patients: a minimal-model analysis. Metabolism 46:880–883
Ader M, Pacini G, Yang YJ, Bergman RN (1985) Importance of glucose per se to intravenous glucose tolerance. Comparison of the minimal-model prediction with direct measurements. Diabetes 34:1092–1103
Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW et al (1994) The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes 43:587–592
Sato Y, Komatsu M, Katakura M, Ohfusa H, Yamada S, Yamauchi K et al (2002) Diminution of early insulin response to glucose in subjects with normal but minimally elevated fasting plasma glucose. Evidence for early beta-cell dysfunction. Diabet Med 19:566–571
Katakura M, Komatsu M, Sato Y, Hashizume K, Aizawa T (2004) Primacy of beta-cell dysfunction in the development of hyperglycemia: a study in the Japanese general population. Metabolism 53:949–953
Aizawa T, Yamada M, Katakura M, Funase Y, Yamashita K, Yamauchi K (2012) Hyperbolic correlation between insulin sensitivity and insulin secretion fades away in lean subjects with superb glucose regulation. Endocr J 59:127–136
Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Accessed at http://whqlibdoc.who.int/publications/2006/9241594934_eng.pdf
Cederholm J, Wibell L (1985) Evaluation of insulin release and relative peripheral resistance with use of the oral glucose tolerance test: a study in subjects with normoglycaemia, glucose intolerance and non-insulin-dependent diabetes mellitus. Scand J Clin Lab Invest 45:741–751
Soonthornpun S, Setasuban W, Thamprasit A, Chayanunnukul W, Rattarasarn C, Geater A (2003) Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab 88:1019–1023
Giannini C, Weiss R, Cali A, Bonadonna R, Santoro N, Pierpont B, Shaw M, Caprio S (2012) Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes 61:606–614
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
DeFronzo RA, Matsuda M (2010) Reduced time points to calculate the composite index. Diabetes Care 33:e93
Kosaka K, Hagura R, Kuzuya T, Kuzuya N (1974) Insulin secretory response of diabetics during the period of improvement of glucose tolerance to normal range. Diabetologia 10:775–782
Kosaka K, Kuzuya T, Hagura R, Yoshinaga H (1996) Insulin response to oral glucose load is consistently decreased in established non-insulin-dependent diabetes mellitus: the usefulness of decreased early insulin response as a predictor of non-insulin-dependent diabetes mellitus. Diabet Med 13(9 Suppl 6):S109–S119
Sokal RR, Rohlf FJ (2011) Biometry, 4th edn. WH Freeman, New York
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327:307–310
Islam MM, Horibe H, Kobayashi F (1999) Current trend in prevalence of diabetes mellitus in Japan, 1964–1992. J Epidemiol 9:155–162
Caumo A, Bergman RN, Cobelli C (2000) Insulin sensitivity from meal tolerance tests in normal subjects: a minimal model index. J Clin Endocrinol Metab 85:4396–4402
Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C (2001) Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 50:150–158
Kanat M, Norton L, Winnier D, Jenkinson C, DeFronzo RA, Abdul-Ghani MA (2011) Impaired early- but not late-phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetol 48:209–217
Gallwitz B, Kazda C, Kraus P, Nicolay C, Schernthaner G (2011) Contribution of insulin deficiency and insulin resistance to the development of type 2 diabetes: nature of early stage diabetes. Acta Diabetol Aug 23 [Epub ahead of print]
Aloulou I, Brun JF, Mercier J (2006) Evaluation of insulin sensitivity and glucose effectiveness during a standardized breakfast test: comparison with the minimal model analysis of an intravenous glucose tolerance test. Metabolism 55:676–690
Brun JF, Ghanassia E, Fédou C, Bordenave S, Raynaud de Mauverger E, Mercier J (2010) Assessment of insulin sensitivity (SI) and glucose effectiveness (SG) from a standardized hyperglucidic breakfast test in type 2 diabetics exhibiting various levels of insulin resistance. Acta Diabetol Oct 28 [Epub ahead of print]
Clausen JO, Borch-Johnsen K, Ibsen H, Bergman RN, Hougaard P, Winther K et al (1996) Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians. Analysis of the impact of gender, body fat, physical fitness, and life-style factors. J Clin Invest 98:1195–1209
Escalante-Pulido M, Escalante-Herrera A, Milke-Najar ME, Alpizar-Salazar M (2003) Effects of weight loss on insulin secretion and in vivo insulin sensitivity in obese diabetic and non-diabetic subjects. Diabetes Nutr Metab 16:277–283
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410
Tokuyama K, Nagasaka S, Mori S, Takahashi N, Kusaka I, Kiyonaga A et al (2009) Hepatic insulin sensitivity assessed by integrated model of hepatic and peripheral glucose regulation. Diabetes Technol Ther 11:487–492
Livesey G, Wilson PD, Dainty JR, Brown JC, Faulks RM, Roe MA et al (1998) Simultaneous time-varying systemic appearance of oral and hepatic glucose in adults monitored with stable isotopes. Am J Physiol 275:E717–E728
Cobelli C, Caumo A, Omenetto M (1999) Minimal model SG overestimation and SI underestimation: improved accuracy by a Bayesian two-compartment model. Am J Physiol 277:E481–E488
Acknowledgments
The study was in part supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (Shoichiro Nagasaka). We thank Masayuki Yamada, Kissei Pharmaceuticals, for invaluable advice regarding statistics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Renato Lauro.
Rights and permissions
About this article
Cite this article
Nagasaka, S., Kusaka, I., Yamashita, K. et al. Index of glucose effectiveness derived from oral glucose tolerance test. Acta Diabetol 49 (Suppl 1), 195–204 (2012). https://doi.org/10.1007/s00592-012-0417-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-012-0417-y