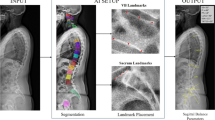
Abstract
Purpose
In past decades, the role of sagittal alignment has been widely demonstrated in the setting of spinal conditions. As several parameters can be affected, identifying the driver of the deformity is the cornerstone of a successful treatment approach. Despite the importance of restoring sagittal alignment for optimizing outcome, this task remains challenging. Self-learning computers and optimized algorithms are of great interest in spine surgery as in that they facilitate better planning and prediction of postoperative alignment. Nowadays, computer-assisted tools are part of surgeons’ daily practice; however, the use of such tools remains to be time-consuming.
Methods: Narrative review and results
Computer-assisted methods for the prediction of postoperative alignment consist of a three step analysis: identification of anatomical landmark, definition of alignment objectives, and simulation of surgery. Recently, complex rules for the prediction of alignment have been proposed. Even though this kind of work leads to more personalized objectives, the number of parameters involved renders it difficult for clinical use, stressing the importance of developing computer-assisted tools. The evolution of our current technology, including machine learning and other types of advanced algorithms, will provide powerful tools that could be useful in improving surgical outcomes and alignment prediction. These tools can combine different types of advanced technologies, such as image recognition and shape modeling, and using this technique, computer-assisted methods are able to predict spinal shape. The development of powerful computer-assisted methods involves the integration of several sources of information such as radiographic parameters (X-rays, MRI, CT scan, etc.), demographic information, and unusual non-osseous parameters (muscle quality, proprioception, gait analysis data). In using a larger set of data, these methods will aim to mimic what is actually done by spine surgeons, leading to real tailor-made solutions.
Conclusion
Integrating newer technology can change the current way of planning/simulating surgery. The use of powerful computer-assisted tools that are able to integrate several parameters and learn from experience can change the traditional way of selecting treatment pathways and counseling patients. However, there is still much work to be done to reach a desired level as noted in other orthopedic fields, such as hip surgery. Many of these tools already exist in non-medical fields and their adaptation to spine surgery is of considerable interest.


Similar content being viewed by others
References
Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F (2005) The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 30(18):2024–2029. https://doi.org/10.1097/01.brs.0000179086.30449.96
Lafage V, Schwab F, Patel A, Hawkinson N, Farcy J-P (2009) Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine (Phila Pa 1976) 34(17):E599–E606. https://doi.org/10.1097/brs.0b013e3181aad219
Aoki Y, Nakajima A, Takahashi H et al (2015) Influence of pelvic incidence-lumbar lordosis mismatch on surgical outcomes of short-segment transforaminal lumbar interbody fusion. BMC Musculoskelet Disord 16(1):213. https://doi.org/10.1186/s12891-015-0676-1
Rothenfluh DA, Mueller DA, Rothenfluh E, Min K (2014) Pelvic incidence-lumbar lordosis mismatch predisposes to adjacent segment disease after lumbar spinal fusion. Eur Spine J. https://doi.org/10.1007/s00586-014-3454-0
Smith JS, Shaffrey CI, Ames CP et al (2012) Assessment of symptomatic rod fracture after posterior instrumented fusion for adult spinal deformity. Neurosurgery 71(4):862–867. https://doi.org/10.1227/NEU.0b013e3182672aab
Moal B, Schwab FJ, Ames CP et al (2014) Radiographic outcomes of adult spinal deformity correction: a critical analysis of variability and failures across deformity patterns. Spine Deform 2(3):219–225. https://doi.org/10.1016/j.jspd.2014.01.003
Hernandez D, Garimella R, Eltorai AEM, Daniels AH (2017) Computer-assisted orthopaedic surgery. Orthop Surg 9(2):152–158. https://doi.org/10.1111/os.12323
Camargo FP, Cordeiro EN, Napoli MM (1986) Corrective osteotomy of the spine in ankylosing spondylitis. Experience with 66 cases. Clin Orthop Relat Res 208:157–167
van Royen BJ, Slot GH (1995) Closing-wedge posterior osteotomy for ankylosing spondylitis. Partial corporectomy and transpedicular fixation in 22 cases. J Bone Joint Surg Br 77(1):117–121
Ondra SL, Marzouk S, Koski T, Silva F, Salehi S (2006) Mathematical calculation of pedicle subtraction osteotomy size to allow precision correction of fixed sagittal deformity. Spine (Phila Pa 1976) 31(25):973–979
Gupta M, Henry JK, Schwab F et al (2016) Dedicated spine measurement software quantifies key spino-pelvic parameters more reliably than traditional picture archiving and communication systems tools. Spine (Phila Pa 1976) 41(1):E22–E37. https://doi.org/10.1097/brs.0000000000001216
Lafage R, Ferrero E, Henry JK et al (2015) Validation of a new computer-assisted tool to measure spino-pelvic parameters. Spine J. 15(12):2493–2502. https://doi.org/10.1016/j.spinee.2015.08.067
Ilharreborde B, Sebag G, Skalli W, Mazda K (2013) Adolescent idiopathic scoliosis treated with posteromedial translation: radiologic evaluation with a 3D low-dose system. Eur Spine J 22(11):2382–2391. https://doi.org/10.1007/s00586-013-2776-7
Ailon T, Scheer JK, Lafage V et al (2016) Adult spinal deformity surgeons are unable to accurately predict postoperative spinal alignment using clinical judgment alone. Spine Deform 4(4):323–329. https://doi.org/10.1016/j.jspd.2016.02.003
Smith JS, Bess S, Shaffrey CI et al (2012) Dynamic changes of the pelvis and spine are key to predicting postoperative sagittal alignment after pedicle subtraction osteotomy: a critical analysis of preoperative planning techniques. Spine (Phila Pa 1976) 37(10):845–853. https://doi.org/10.1097/brs.0b013e31823b0892
Lafage V, Schwab F, Vira S, Patel A, Ungar B, Farcy J-P (2011) Spino-pelvic parameters after surgery can be predicted a preliminary formula and validation of standing alignment. Spine (Phila Pa 1976) 36(13):1037–1045. https://doi.org/10.1097/brs.0b013e3181eb9469
Langella F, Villafane JH, Damilano M et al (2017) Predictive accuracy of SurgimapTM surgical planning for sagittal imbalance: A Cohort Study. Spine (Phila Pa 1976). https://doi.org/10.1097/brs.0000000000002230
Shea KG, Stevens PM, Nelson M, Smith JT, Masters KS, Yandow S (1998) A comparison of manual versus computer-assisted radiographic measurement. Intra observer measurement variability for Cobb angles. Spine (Phila Pa 1976) 23(5):551–555
Terran J, Schwab FJ, Shaffrey CI et al (2013) The SRS-Schwab adult spinal deformity classification: assessment and clinical correlations based on a prospective operative and nonoperative cohort. Neurosurgery 73(4):559–568. https://doi.org/10.1227/NEU.0000000000000012
Lafage R, Schwab F, Challier V et al (2016) Defining spino-pelvic alignment thresholds: should operative goals in adult spinal deformity surgery account for age? Spine (Phila Pa 1976) 41(1):62–68. https://doi.org/10.1097/brs.0000000000001171
Yilgor C, Sogunmez N, Boissiere L et al (2017) Global alignment and proportion (GAP) score: development and validation of a new method of analyzing spinopelvic alignment to predict mechanical complications after adult spinal deformity surgery. J Bone Joint Surg Am 99:1661–1672. https://doi.org/10.2106/JBJS.16.01594
Benjelloun M, Mahmoudi S (2009) Spine localization in X-ray images using interest point detection. J Digit Imaging 22:309–318. https://doi.org/10.1007/s10278-007-9099-3
Scheer JK, Smith JS, Schwab F et al (2017) Development of preoperative predictive model for major complications following adult spinal deformity surgery. J Neurosurg Spine 26:736–743. https://doi.org/10.3171/2016.10.SPINE16197
Benjelloun M, Mahmoudi S, Lecron F (2011) A framework of vertebra segmentation using the active shape model-based approach. Int J Biomed Imaging 2011:621905. https://doi.org/10.1155/2011/621905
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential conflict of interest.
Rights and permissions
About this article
Cite this article
Lafage, R., Pesenti, S., Lafage, V. et al. Self-learning computers for surgical planning and prediction of postoperative alignment. Eur Spine J 27 (Suppl 1), 123–128 (2018). https://doi.org/10.1007/s00586-018-5497-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5497-0