Abstract
Purpose
To quantitatively synthesize the available best evidence for general complications, heterotopic ossification (HO), retrograde ejaculation, cervical swelling, and cancer rates with the use of rhBMP-2 in lumbar and cervical spine fusion.
Methods
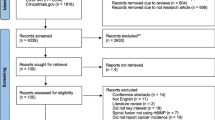
We conducted an online search for relevant controlled trials and extracted data on the abovementioned endpoints. Studies were eligible for inclusion if they reported on spinal fusion with rhBMP-2 in humans. Publication bias and heterogeneity were assessed mathematically. These data were synthesized in a meta-analysis using DerSimonian–Laird random effects modeling to calculate pooled odds ratios.
Results
We identified 26 studies reporting on a total of 184,324 patients (28,815 experimental, 155,509 controls) with a mean age of 51.1 ± 1.8 years. There was a significantly higher risk of general complications with rhBMP-2 compared to iliac crest bone graft (ICBG) with an odds ratio (OR) of 1.78 (95 %CI 1.20–2.63), (p = 0.004). The odds ratio for HO was 5.57 (95 %CI 1.90–16.36), (p = 0.002), for retrograde ejaculation 3.31 (95 %CI 1.20–9.09), (p = 0.020), and for cervical swelling 4.72 (95 %CI 1.42–15.67), (p = 0.011), all significantly higher in the rhBMP-2 group. The pooled odds ratio for new onset of tumor was 1.35 (95 %CI 0.93–1.96), which represents no statistically significant difference between the groups (p = 0.111).
Conclusion
rhBMP-2 is associated with a higher rate of general complications as well as retrograde ejaculation, HO, and cervical tissue swelling in spine fusion. There is a slightly increased risk of new onset of tumors, however, without statistical significance.






Similar content being viewed by others
References
Boden SD, Kang J, Sandhu H, Heller JG (2002) Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial 2002 Volvo award in clinical studies. Spine 27:2662–2673
Boden SD, Zdeblick TA, Sandhu HS, Heim SE (2000) The use of rhBMP-2 in interbody fusion cages: definitive evidence of osteoinduction in humans: a preliminary report. Spine 25:376–381
Singh K, Nandyala SV, Marquez-Lara A et al (2013) Clinical sequelae after rhBMP-2 use in a minimally invasive transforaminal lumbar interbody fusion. Spine J 13:1118–1125. doi:10.1016/j.spinee.2013.07.028
Veeravagu A, Cole TS, Jiang B et al (2014) The use of bone morphogenetic protein in thoracolumbar spine procedures: analysis of the MarketScan longitudinal database. Spine J. doi:10.1016/j.spinee.2014.05.010
Fu R, Selph S, McDonagh M et al (2013) Effectiveness and Harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med 158:890–902. doi:10.7326/0003-4819-158-12-201306180-00006
Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA (2002) Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. doi:10.1097/01.BRS.0000030193.26290.DD
Baskin DS, Ryan P, Sonntag V et al (2003) A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR™ allograft ring and the ATLANTIS™ anterior cervical plate. Spine 28:1219–1224. doi:10.1097/01.BRS.0000065486.22141.CA
Johnsson R, Strömqvist B, Aspenberg P (2002) Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion: 2002 Volvo Award in Clinica. Stud Spine 27:2654–2661
Cammisa FP Jr, Lowery G, Garfin SR, Geisler FH (2004) Two-year fusion rate equivalency between Grafton® DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side Comparison in the Same Patient. Spine 29:660–666
Tannoury CA, An HS (2014) Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 14:552–559. doi:10.1016/j.spinee.2013.08.060
Mesfin A, Buchowski JM, Zebala LP et al (2013) High-dose rhBMP-2 for adults: major and minor complications. J Bone Joint Surg Am 95:1546–1553. doi:10.2106/JBJS.L.01730
Joseph V, Rampersaud YR (2007) Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion. Spine 32:2885–2890. doi:10.1097/BRS.0b013e31815b7596
Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK (2004) Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J 4:527–538. doi:10.1016/j.spinee.2004.03.025
Mannion RJ, Nowitzke AM, Wood MJ (2011) Promoting fusion in minimally invasive lumbar interbody stabilization with low-dose bone morphogenic protein-2—but what is the cost? Spine J 11:527–533. doi:10.1016/j.spinee.2010.07.005
Comer GC, Smith MW, Hurwitz EL et al (2012) Retrograde ejaculation after anterior lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: a 10-year cohort controlled study. Spine J 12:881–890. doi:10.1016/j.spinee.2012.09.040
Carragee EJ, Mitsunaga KA, Hurwitz EL, Scuderi GJ (2011) Retrograde ejaculation after anterior lumbar interbody fusion using rhBMP-2: a cohort controlled study. Spine J 11:511–516. doi:10.1016/j.spinee.2011.02.013
Burkus JK, Dryer RF, Peloza JH (2013) Retrograde ejaculation following single-level anterior lumbar surgery with or without recombinant human bone morphogenetic protein–2 in 5 randomized controlled trials. J Neurosurg Spine 18:112–121. doi:10.3171/2012.9.SPINE12476
Cahill KS, Chi JH, Day A, Claus EB (2009) Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 302:58–66. doi:10.1001/jama.2009.956
Hiremath GK, Steinmetz MP, Krishnaney AA (2009) Is it safe to use recombinant human bone morphogenetic protein in posterior cervical fusion? Spine 34:885–889. doi:10.1097/BRS.0b013e31819e334a
Smucker JD, Rhee JM, Singh K et al (2006) Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine 31:2813–2819. doi:10.1097/01.brs.0000245863.52371.c2
Buttermann GR (2008) Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J 8:426–435. doi:10.1016/j.spinee.2006.12.006
Vaidya R, Carp J, Sethi A et al (2007) Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J 16:1257–1265. doi:10.1007/s00586-007-0351-9
Carragee EJ, Chu G, Rohatgi R et al (2013) Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am 95:1537. doi:10.2106/JBJS.L.01483
Simmonds MC, Brown JVE, Heirs MK et al (2013) Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med 158:877–889. doi:10.7326/0003-4819-158-12-201306180-00005
Moher D, Altman DG, Liberati A, Tetzlaff J (2011) PRISMA statement. Epidemiology 22:128–133. doi:10.1097/EDE.0b013e3181fe7825
Moher D, Cook DJ, Eastwood S et al (1999) Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet 354:1896–1900. doi:10.1016/S0140-6736(99)04149-5
Hoffmann MF, Jones CB, Sietsema DL (2013) Complications of rhBMP-2 utilization for posterolateral lumbar fusions requiring reoperation: a single practice, retrospective case series report. Spine J 13:1244–1252. doi:10.1016/j.spinee.2013.06.022
Pradhan BB, Bae HW, Dawson EG et al (2006) Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein-2. Spine 31:E277–E284. doi:10.1097/01.brs.0000216442.12092.01
Mummaneni PV, Pan J, Haid RW, Rodts GE (2004) Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report: invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine 1:19–23. doi:10.3171/spi.2004.1.1.0019
Jadad AR, Enkin MW (2008) Randomized Controlled Trials. John Wiley & Sons, Newyork
Vavken P, Dorotka R (2009) A systematic review of conflicting meta-analyses in orthopaedic surgery. Clin Orthop Relat Res 467:2723–2735. doi:10.1007/s11999-009-0765-2
Egger M, Smith GD, Altman D (2001) Systematic reviews in health care. BMJ Books
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. doi:10.1002/sim.1186
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. doi:10.1016/0197-2456(86)90046-2
Burkus JK, Gornet MF, Dickman CA, Zdeblick TA (2002) Anterior lumbar interbody fusion using rhBMP-2 With tapered interbody cages. J Spinal Disord Tech 15:337–349
Schultz D, U.S. Food and Drug Administration, Department of Health and Human Services, Center for Devices and Radiological Health. In: FUSETM Bone Graft/LT-CAGETM Lumbar Tapered Fusion Devices—P000058. Summary of safety and effectiveness data. 2002. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf/P000058b.pdf. Accessed 14 Feb 2010
Slosar PJ, Josey R, Reynolds J (2007) Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J 7:301–307. doi:10.1016/j.spinee.2006.10.015
Carreon LY, Glassman SD, Djurasovic M et al (2009) RhBMP-2 versus iliac crest bone graft for lumbar spine fusion in patients over 60 years of age: a cost-utility study. Spine 34:238–243. doi:10.1097/BRS.0b013e31818ffabe
Dawson E, Bae HW, Burkus JK, Stambough JL (2009) Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive bulking agent in posterolateral arthrodesis with instrumentation. J Bone Joint Surg Am 91:1604–1613. doi:10.2106/JBJS.G.01157
DimarII JR (2009) Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am 91:1377–1386. doi:10.2106/JBJS.H.00200
Rihn JA, Patel R, Makda J et al (2009) Complications associated with single-level transforaminal lumbar interbody fusion. Spine J 9:623–629. doi:10.1016/j.spinee.2009.04.004
Latzman JM, Kong L, Liu C, Samadani U (2010) Administration of human recombinant bone morphogenetic protein-2 for spine fusion may be associated with transient postoperative renal insufficiency. Spine 35:E231–E237. doi:10.1097/BRS.0b013e3181c71447
Lindley EM, McBeth ZL, Henry SE, Cooley R (2012) Retrograde ejaculation after anterior lumbar spine surgery. Spine 20:1785–1789. doi:10.1097/BRS.0b013e31825752bc
Cooper GS, Kou TD (2013) Risk of cancer following lumbar fusion surgery with recombinant human bone morphogenic protein-2 (rh-BMP-2). Spine 38:1862–1868. doi:10.1097/BRS.0b013e3182a3d3b4
Hurlbert RJ, Alexander D, Bailey S et al (2013) rhBMP-2 for Posterolateral Instrumented Lumbar Fusion. Spine 38:2139–2148. doi:10.1097/BRS.0000000000000007
Lad SP, Bagley JH, Karikari IO, Babu R (2013) Cancer after spinal fusion: the role of bone morphogenetic protein. Neurosurgery 73:440–449. doi:10.1227/NEU.0000000000000018
Pimenta L, Marchi L, Oliveira L et al (2013) A prospective, randomized, controlled trial comparing radiographic and clinical outcomes between stand-alone lateral interbody lumbar fusion with either silicate calcium phosphate or rh-BMP2. J Neurol Surg A Cent Eur Neurosurg 74:343–350. doi:10.1055/s-0032-1333420
Tepper G, Rabbani R, Yousefzadeh M, Prince D (2013) Quantitative assessment of retrograde ejaculation using semen analysis, comparison with a standardized qualitative questionnaire, and investigating the impact of rhBMP-2. Spine 38:841–845. doi:10.1097/BRS.0b013e31828bf36a
Ong KL, Villarraga ML, Lau E et al (2010) Off-label use of bone morphogenetic proteins in the united states using administrative data. Spine 35:1794–1800. doi:10.1097/BRS.0b013e3181ecf6e4
Muchow RD, Hsu WK, Anderson PA (2010) Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J 10:e1–e6. doi:10.1016/j.spinee.2010.06.020
Stanton T (2015) Will YODA end debate over rhBMP-2? 1–6. AAOS Now August 2013 Issue. http://www.aaos.org/news/aaosnow/aug13/cover1.asp
Weiner BK, Hurwitz EL, Schoene ML (2013) Moving forward after YODA. Spine J 13:995–997. doi:10.1016/j.spinee.2013.08.001
Thomas E, Mroz Jeffrey C, Wang Robin Hashimoto, Daniel C, Norvell P (2014) Complications Related to osteobiologics use in spine surgery. Spine 9S:86–104. doi:10.1097/BRS.0b013e3181d81ef2
Siemionow K, Sundberg E, Tyrakowski M et al (2014) Point-counter-point debate: the association between recombinant human bone morphogenetic protein utilization and complications in spine surgery. Curr Rev Musculoskelet Med 7:200–207. doi:10.1007/s12178-014-9219-x
Rihn JA, Makda J, Hong J et al (2009) The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: a clinical and radiographic analysis. Eur Spine J 11:1629–1636. doi:10.1007/s00586-009-1046-1
Sasso RC, Kenneth Burkus J, LeHuec J-C (2003) Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine 28:1023–1026
Smoljanovic T, Rakovac M, Bojanic I (2011) Could chronic host inflammatory response be responsible for delayed onset of retrograde ejaculation after the labeled use of recombinant human bone morphogenetic protein-2? Spine J 11:167–168. doi:10.1016/j.spinee.2010.10.024
Klimo P, Peelle MW (2009) Use of polyetheretherketone spacer and recombinant human bone morphogenetic protein-2 in the cervical spine: a radiographic analysis. Spine J 9:959–966. doi:10.1016/j.spinee.2009.05.008
Administration FAD (2008) FDA public health notification: life-threatening complications associated with recombinant human bone morphogenetic protein in cervical spine fusion. Available at: http://www.spine.org/Documents/rhBMP_cervical_7-08_rev%20_2_pdf
Benglis D, Wang MY, Levi AD (2014) A comprehensive review of the saftey profile of bone morphogenetic protein on spine surgery. Neurosurgery 62:423–431. doi:10.1227/01.NEU.0000297139.47969.14
Mehler MF, Mabie PC, Zhang D, Kessler JA (1997) Bone morphogenetic proteins in the nervous system. Trends Neurosci 20:309–317. doi:10.1007/978-3-0348-7857-9_11
Müller MA, Mehrkens A, Zürcher R et al (2014) Effectiveness of the addition of Lidocaine to a hemostatic, bioresorbable putty in the treatment of iliac crest donor site pain. BMC Musculoskelet Disord 15:415. doi:10.1186/1471-2474-15-415
Schwartz CE, Martha JF, Kowalski P et al (2009) Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual Life Outcome 7:49–56. doi:10.1186/1477-7525-7-49
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vavken, J., Mameghani, A., Vavken, P. et al. Complications and cancer rates in spine fusion with recombinant human bone morphogenetic protein-2 (rhBMP-2). Eur Spine J 25, 3979–3989 (2016). https://doi.org/10.1007/s00586-015-3870-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-3870-9