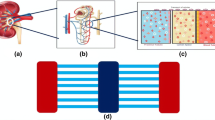
Abstract
The flow in the nephron tubules regulates the blood homeostasis, secretion, excretion and reabsorption of solutes. Flow induces fluid shear stress (FSS) on walls of tubules which has substantial effect on kidney functioning. Modelling of FSS mimicking structures is critical for their optimization. In this paper, a compact structure for kidney-on-chip devices is proposed for exact reconstruction of FSS and temperature effect in tubules considering the nephron tubule dimensions for modelling. The proposed system is compartmentalized basically into three parts ultra filter, device structure and reactor. The FSS is analysed for different inflow rates, proving the proposed structure is helpful in balancing the tubuloglomerulo feedback system mimicking. The structure is analysed for different structural changes in benchmark FEM tool COMSOL Multiphysics to optimize it to suite the kidney-on-chip purpose. Pressure achieved at the cell site is 0.1 Pa, which is the pressure applied by FFS on kidney tubules cells. The temperature effect of the human body is considered and the findings are presented. Temperature has an inverse relationship with FSS. At absolute temperature the pressure is 9.7 Pa and reached 3.77 Pa at body temperature, showing a decrease in pressure. The reaction of sodium and calcium is presented to show common reabsorption happening the kidney. The fabrication process flow is implemented in Intellisuite tool which is incorporated here to indicate the device fabrication cost will be low. The proposed Kidney-on-Chip structure finds astounding results make it to use in potential transplantable devices.











Similar content being viewed by others
References
Adams T, Yang C, Gress J, Wimmer N, Minerick AR (2012) Chapter: 9 “Advances in Microfluidics”. Intech Publishing, Den Haag
Al-Awqati Q (2012) “Structure and function of the kidneys”, Goldman’s cecil medicine, 24th edn. Elsevier Saunders, Philadelphia, pp 716–720
Bhise NS, Ribas J, Manoharan V, Zhang YS, Polini A, Massa S, Dokmeci MR, Khademhosseini A (2014) Organ-on-a-chip platforms for studying drug delivery systems. J Control Release 190:82–93
Cai Z, Xin J, Pollock DM, Pollock JS (2000) Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol 279:F270–F274
Couser WG, Remuzzi G, Mendis S, Tonelli M (2011) The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80(12):1258–1270
Davies PF (1997) Overview: temporal and spatial relationships in shear stressmediated endothelial signaling. J Vasc Res 34:208–211
Dewey CF, Bussolari SR, Gimbrone MA, Davies PF (1981) The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103:177–185
Donvito L, Galluccio L, Lombardo A, Morabito G, Nicolosi A, Reno M (2015) Experimental validation of a simple, low-cost, T-junction droplet generator fabricated through 3D printing. J Micromech Microeng 25:035013
Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T (2004) Mechnosensory function of microvilli of the kidney proximal tubule. PNAS 101:13068–13073
Duan Y, Gotoh N, Yan Q, Du Z, Weinstein AM, Wang T, Weinbaum S (2008) Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. PNAS 105:11418–11423
Essig M, Terzi F, Burtin M, Friedlander G (2001) Mechanical strains induces by tubular flow affect the phenotype of proximal tubular cells. Am J Physiol Renal Physiol 281:F751–F762
Ferrell N, Cheng J, Miao S, Roy S, Fissell WH (2017) Orbital shear stress regulates differentiation and barrier function of primary renal tubular epithelial cells. ASAIO J 64(6):766–772. https://doi.org/10.1097/MAT.0000000000000723
Haiden C, van den Driesche S, Iuliano F, Vellekoop MJ (2011) A microfluidic chip for infrared CH2-stretch ratio measurements of suspended mammalian cells. Proceedings GMe Forum 2011
Humes HD, Buffington D, Westover AJ, Roy S, Fissell WH (2014) The bioartificial kidney: current status and future promise. Pediatr Nephrol 29(3):343–351
Jang KJ, Suh KY (2009) A multi-layer microfluidic device for eficient culture and analysisi of renal tubular cells. Lab Chip 10:36–42
Kidney Disease (2013) Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter 3(1)(suppl):1–150
Kim S, Roy S (2013) Microelectromechanical systems and nephrology: the next frontier in renal replacement technology. Adv Chronic Kidney Dis 20(6):516–535
Kim S, Fissell WH, Humes DH, Roy S (2015) Current strategies and challenges in engineering a bioartificial kidney. Front Biosci (Elite Ed) 7:215–228
Laffite G, Leroy C, Bonhomme C, Bonhomme-Coury L, Letavernier E, Daudon M, Frochot V, Haymann JP, Rouzière S, Lucas IT, Bazin D, Babonneau F, Abou-Hassan A (2016) Calcium oxalate precipitation by diffusion using laminar microfluidics: toward a biomimetic model of pathological microcalcifications. Lab Chip 16:115–1160
Li X, Wei Z, Zhao D, Yan H, Liang Z, Li Z (2012) A flow-through electroporation chip integrated with viable cell sorting based on dielectrophoresis. MEMS, Paris, pp 993–996
Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM (2003) Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285:F998–F1012
Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM (2005) Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289:F978–F988
Malic L, Brassard D, Veres T, Tabrizian M (2010) Integration and detection of biochemical assays in digital microfluidic LOC devices. Lab Chip 10:418–431
Mu X, Zheng W, Xiao L, Zhang W, Jiang X (2013) Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip. https://doi.org/10.1039/c3lc41342j
Raghavan V, RabaibiV Pastor-Soler NM, Carattino MD, Weisz OA (2014) Shear stres-dependent regulation of apical endocyotsisin renal proximal tubule cells mediated by primary cilia. Proc Natl Acad Sci USA 111:8506–8511
Richards IS (2008) Principles and practice tocicology in public healt. Jones and Bartlell Publishers, Sudbury
Ruige W, Fung YS (2015) Microfluidic chip-capillary electrophoresis device for the determination of urinary metabolites and proteins. Bioanalysis 7:907–922
Sarirstein RL (2004) Acute renal failure: from renal physiology to renal transcriptome. Kidney Intern 66:s62–s66
Sateesh J, Sravani KG, Kumar RA, Guha K, Rao KS (2017) Design and flow analysis of MEMS based piezo-electric micro pump. Microsyst Technol. https://doi.org/10.1007/s00542-017-3563-x
Sia SK, Kricka LJ (2008) Microfluidics and point-of-care testing. Lab Chip 8:1982–1983
Sochol RD, Gupta NR, Bonventre JV (2016) A role for 3D printing in kidney-on-a-chip platforms. Curr Transpl Rep 3(1):82–92
Suwanpayak N, Jalil MA, Aziz MS, Ismail FD, Ali J, Yupapin PP (2011) Blood clenar on-chip design for artificial human kidney manipulation. Int J Nanomed 6:957–964
Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437:426–431
Wang L, Tao T, Su W, Yu H, Yu Y, Qin J (2017) A desease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip 17:1749–1760
Zhang R, Jullien GA, Dalton C (2013) Study on an alternating current electrothermal micropump for microneedlebased fluid delivery systems. J Appl Phys 114:024701
Zheng W, Wang Z, Zhang W, Jiang X (2010) A simple PDMS-based microfluidic channel design that removes bubles for lon-term on-chip culture of mammalian cells. Lab Chip 10:2906–2910
Acknowledgments
The authors earnestly like to thank to National MEMS Design Center supported by National Institute of Technology (NMDC @ NIT Silchar), Silchar for providing the financial assistance and computer tools (COMSOL Multiphysics and Intellisuite Fab).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sateesh, J., Guha, K., Dutta, A. et al. Design and analysis of microfluidic kidney-on-chip model: fluid shear stress based study with temperature effect. Microsyst Technol 25, 2553–2560 (2019). https://doi.org/10.1007/s00542-018-4261-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-018-4261-z