Abstract
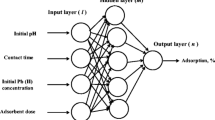
In this study, the nanoparticles of sol–gel-synthesized NiO were used as effective adsorbents for removing Cr(VI) from aqueous solutions. To do so, the effect of four initial parameters including Cr(VI) concentration, the amount of NiO adsorbent, contact time, and pH on removing Cr(VI) with sol–gel-synthesized NiO was studied. Using the results of designing the experiment, the process of surface adsorption by ANN was modelled. For modelling the results of Cr(VI) removal process with NiO nanoparticles, a three-layered ANN of feed-forward back-propagation having 4:10:1 topology was used. The findings indicated that the results obtained from ANN correspond well with the data obtained from response surface methodology and experimental data.














Similar content being viewed by others
Change history
10 January 2018
The author list in the original publication included Mohammad A. Behnajady as second author.
References
Ahmadia F, Valadan Zoeja MJ, Ebadia H, Mokhtarzadea M (2008) The application of neural networks, image processing and cad—based environments facilities in automatic road extraction and vectorization from high resolution satellite images. In: The international archives of the photogrammetry, remote sensing and spatial information sciences, vol XXXVII. Part B3b, pp 585–592, Beijing
Aleboyeh A, Kasiri MB, Olya ME, Aleboyeh H (2008) Prediction of azo dye decolorization by UV/H2O2 using artificial neural networks. Dyes Pigm 72:288–294. doi:10.1016/jdyepig.2007.05.014
Amrouche A, Debyeche M, Taleb-Ahmed A, Rouvaen MJ, Yagoub M (2008) An efficient speech recognition system in adverse conditions using the nonparametric regression. Eng Appl Artif Intell 23:85–94. doi:10.1016/j.engappai.2009.09.006
Behin J, Farhadian N (2016) Response surface methodology and artificial neural network modeling of reactive red 33 decolorization by O3/UV in a bubble column reactor. Adv Environ Technol 1(2016):33–44
Cheok CY, Chin NL, Yusof YA, Talib RA, Law CL (2012) Optimization of total phenolic content extracted from Garcinia mangostana Linn. Hull using response surface methodology versus artificial neural network. Ind Crops Prod 40:247–253. doi:10.1016/j.indcrop
Cheung CW, Porter JF, Mckay G (2001) Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Res 35:605–612. doi:10.1016/S0043-1354(00)00306-7
Daneshvar N, Khataee AR, Djafarzadeh N (2006) The use of artificial neural networks (ANN) for modeling of decolorization of textile dye solution containing C.I. Basic Yellow 28 by electrcoagulation process. J Hazard Mater B 137:1788–1795. doi:10.1016/j.jhazmat.2006.05.042
Ebrahimzadeh H, Tavassoli N, Sadeghi O, Amini MM (2012) Optimization of solid-phase extraction using artificial neural networks and response surface methodology in combination with experimental design for determination of gold by atomic absorption spectrometry in industrial wastewater samples. Talanta 97:211–217. doi:10.1016/j.talanta.2012.04.019
Ghafari S, Abdul Aziz H, Hasnain Isa M, Zinatizadeh AA (2009) Application of response surface methodology (RSM) to optimize coagulation–flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J Hazard Mater 163:650–656. doi:10.1016/j.jhazmat.2008.07.090
Gonen F, Serin S (2012) Adsorption study on orange peel: removal of Ni(II) ions from aqueous solution. Afr J Biotechnol 11:1250–1258. doi:10.5897/AJB11.1753
Gupta VK, Suhas S (2009) Application of low-cost adsorbents for dye removal—a review. J Environ Manage 90:2313–2342. doi:10.1016/j.jenvman.2008.11.017 (get rights and content)
Hamed MM, Khalafallah MG, Hassanien EA (2004) Prediction of wastewater treatment plant performance using artificial neural network. Environ Modell Softw 19:919–928. doi:10.1155/2013/268064
Hu J, Chen G, Lo IMC (2005) Removal & recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res 39:4528–4536. doi:10.1016/j.watres.2005.05.051
Khajeh M, Kaykhaii M, Sharafi A (2013) Application of PSO-artificial neural network and response surface methodology for removal of methylene blue using silver nanoparticles. J Ind Eng Chem 19(5):1624–1630. doi:10.1016/j.jiec.2013.01.033
Khataee AR, Zarei M, Pourhassan M (2009) Application of microalga chlamydonas sp. For biosorptive removal of a textile dye from contaminated water: modeling by a neural network. Environ Technol 30:1615–1623. doi:10.1080/09593330903370018
Khayet M, Cojocaru C (2013) Artificial neural network model for desalination by sweeping gas membrane distillation. Desalination 308:102–110. doi:10.1016/j.desal.2012.06.023
Kia SM Soft Computing in matlab. Kian Rayaneh Sabz
Krisha R, Padma S (2013) Artificial neural network and response surface methodology approach for modeling and optimization of chromium (VI) adsorption from waste water using Ragi husk powder. Indian Chem Eng. doi:10.1080/00194506.2013.829257
Lakshminarayanan AK, Balasubramanian V (2009) Comparison of RSM with ANN in predicting tensile strength of friction stir welded AA7039 aluminium alloy joints. Trans Nonferr Metals Soc China 19(1):9–18. doi:10.1016/S1003-6326(08)60221-6
Mahmood T, Saddique MT, Naeem A, Mustafa S, Hussain J, Dilara B (2011) Cation exchange removal of Zn from aqueous solution by NiO. J Non Cryst Solids 357:1016–1020. doi:10.1016/j.jnoncrysol.2010.11.044
Modirshahla N, Behnajady MA, Shamel A, Eskandari H (2010) Sorption study of C.I. Acid Red 88 from aqueous solutions onto sawdust. J Phys Theor Chem IAU Iran 7(2):77–81
Moussavi G, Mahmoudi M (2009) Removal of azo & anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J Hazard Mater 168:806–812. doi:10.1016/j.jhazmat.2009.02.097
Nandi BK, Goswami A, Purkai MK (2009) Adsorption characteristics of brilliant green dye on kaolin. J Hazard Mater 161:387–395. doi:10.1016/j.jhazmat
Niaei A, Towfighi J, Khataee AR, Rostamizadeh K (2007) The use of ANN and mathematical model for prediction of main product yields in the thermal cracking of naphtha. Pet Sci Technol 25:967–982. doi:10.1080/10916460500423304
Oubagaranadin JUK and Murthy Z (2009) Modeling of adsorption of chromium (VI) on activated carbons derived from corn (zeamays) cob. Chem Prod Process Model 4, Article 32. doi:10.2202/1934-2659.1377
Ozacar M, Sengil IA (2005) Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour Technol 96:791–795. doi:10.1016/j.biortech.2004.07.011
Qamar M, Gondal MA, Yamani ZH (2011) Synthesis of nanostructured NiO and its application in laser-induced photocatalytic reduction of Cr(VI) from water. J Mol Catal A 341:83–88. doi:10.1016/j.molcata.2011.03.029
Rao KS, Anand S, Rout K, Venkatesewarlu P (2012) Response surface optimization for removal of cadmium from aqueous solution by waste agricultural biosorbent Psidium guvajava L. leaf powder. CLEAN Soil Air Water 40:80–86. doi:10.1002/clen.201000392
Sinha K, Saha PD, Datta S (2012) Response surface optimization and artificial neural network modeling of microwave assisted natural dye extraction from pomegranate rind. Ind Crops Prod 37(1):408–414. doi:10.1016/j.indcrop.2011.12.032
Xiang L, Deng XY, Jin Y (2002) Experimental study on synthesis of NiO nano-particles. Scr Mater 47:219–224. doi:10.1016/S1359-6462(02)00108-2
Yetilmezsoy K, Demirel S (2008) Artificial neural network (ANN) approach for modeling of Pb(II) adsorption from aqueous solution by Antep pistachio (Pistacia vera L.) shells. J Hazard Mater 153(2008):1288–1300. doi:10.1016/j.jhazmat.2007.09.092
Ziaeifar N, Khosravi M, Behnajady MA, Mahmood R, Sohrabi MR, Modirshahla N (2015) Optimizing adsorption of Cr(VI) from aqueous solutions by NiO nanoparticles using aguchi and response surface methods. Water Sci Technol. doi:10.2166/wst.2015.253
Acknowledgements
The authors gratefully acknowledge their appreciation to the Islamic Azad University, Maragheh, for providing facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
The original version of this article was revised: The author, Mohammad A. Behnajady was wrongly included as second author in the author list. This was incorrect. The correct author list is as follows: Saber Khodaei Ashan, Nasim Ziaeifar and Rana Khalilnezhad. Now, it has been corrected.
Rights and permissions
About this article
Cite this article
Ashan, S.K., Ziaeifar, N. & Khalilnezhad, R. Artificial neural network modelling of Cr(VI) surface adsorption with NiO nanoparticles using the results obtained from optimization of response surface methodology. Neural Comput & Applic 29, 969–979 (2018). https://doi.org/10.1007/s00521-017-3172-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-017-3172-8