Abstract
Purpose
Aromatase inhibitors are standard of care in women with hormone receptor-positive early breast cancer. Published evidence demonstrates that adverse effects may have an impact on drug compliance, with arthralgias being one of the most commonly reported adverse effects.
Methods
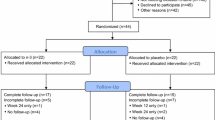
Eligible patients were postmenopausal women who had experienced arthralgia following initiation of an aromatase inhibitor. Patients who experienced arthralgias following a minimum of a 3-month treatment on the aromatase inhibitor were randomized to emu oil or placebo oil. The primary endpoint was to assess for a reduction in pain as measured by a visual analogue score after 8 weeks of treatment.
Results
Seventy-three patients comprised the intent-to-treat population, and there was no statistically significant benefit with use of EO. However, there was a statistically significant improvement in pain (visual analogue score was −1.28; p < 0.001) and Brief Pain Inventory severity score −0.88 (p < 0.001), as well as functional interference (Brief Pain Inventory interference −1.10 (p < 0.001) for the entire population following an 8-week administration of EO or placebo oil.
Conclusions
Arthralgias, as a result of aromatase inhibitor use, may be ameliorated by the use of topical oil massaged onto the joint. Further research into interventions for this common side effect is needed.

Similar content being viewed by others
References
The Arimidex, Tamoxifen, alone or in combination Trialists’ Group, Buzdar A, Howell A, Cuzick J, Wale C, Distler W, Hoctin-Boes G, Houghton J, Locker G, Nabholtz J et al (2006) Comprehensive side effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol. 7(8):633–643
Goss P, Ingle J, Martino S et al (2003) A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 349:1793–1802
Coombes R, Kilburn L, Snowdon C et al. (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomized controlled trial. Lancet 559–570
The Breast International Group (Big) (2005) 1–98 Collaborative Group et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2747–2757
Sestak I, Cuzick J, Sapunar F et al (2008) Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol.:866–872
Kaufmann M, Walter Jonat, Jörn Hilfrich, Holger Eidtmann, Günther Gademann, Ivan Zuna, Gunter Von Minckwitz et al (2007) Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95. Study J Clin Oncol 2664–2670
Henry N, Azzouz F, Desta Z et al (2012) Predictors of AI discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J. Clin. Oncol. 30:936–942
Burstein H And Eric P. (2007) Winer et al. Aromatase inhibitors and arthralgias: a new frontier in symptom management for breast cancer survivors. J Clin Oncol (Editorial) 25:3797–3799
Crew K, Capodice J, Greenlee H et al (2010) Randomized, blinded, sham-controlled trial of acupuncture for the management of AI-associated joint symptoms in women with early-stage breast cancer. J. Clin. Oncol.:1154–1160
Greenlee H, Crew KD, Shao T, Kranwinkel G, Kalinsky K, Maurer M, Phase II (2013 Apr) Study of glucosamine with chondroitin on AI-associated joint symptoms in women with breast cancer. Support Care Cancer 21(4):1077–1087
Irwin M, Cartmel B, Gross C et al (2015) Randomized exercise trial of AI-induced arthralgia in breast cancer survivors. J. Clin. Oncol. 33:1104–1111
Power R, Cameron M. Emu oil for osteoarthritic hand pain at http://www.emuindustry.asn.au/ RayPowerClinicalTrial.htm
Lombard J, Zdenkowski N, Wells K, Beckmore C, Reaby L, Forbes J, Chirgwin J et al (2016) AI induced musculoskeletal syndrome: a significant problem with limited treatment options. Support Care Cancer 24:2139–2146
Kadakia K, Snyder C, Kidwell K, Seewald N, Flockhart D, Skaar T, Desta Z, Rae J, Otte J, Carpenter J, Storniolo A, Hayes D, Stearns V, Henry N et al (2016) Patient-reported outcomes and early discontinuation in AI-treated postmenopausal women with early stage breast cancer. Oncologist 21:539–546
Morales L, Pans S, Verschueren K, Van Calster B, Paridaens R, Westhovens R, Timmerman D, De Smet L, Vergote I, Christiaens Mr, Neven P. et al (2008) Prospective study to assess short-term intra-articular and tenosynovial changes in the AI-associated arthralgia syndrome. J Clin Oncol 26
Lintermans A, Laenen A, Van Calster B, Van Hoydonck M, Pans S, Verhaeghe J, Westhovens R, Nl H, Wildiers H, Paridaens R, Dieudonné AS, Leunen K, Morales L, Verschueren K, Timmerman D, De Smet L, Vergote I, Christiaens M, Neven P et al (2013) Arthralgia and changes in serum levels of IGF-I, its binding protein and oestrogen in breast cancer patients on endocrine agents. Ann. Oncol. 24:350–355
Yoganathan S, Nicolosi R, Wilson T et al (2006) Antagonism of croton oil inflammation by topical emu oil in CD-1 mice. Lipids 38:603–607
Qiu X, Wang J, Fang X et al (2005) Anti-inflammatory activity and healing-promoting effects of topical application of emu oil on wound in scalded rats. Di Yi Jun Yi Da Xue Xue Bao 25(4):407–410
Perlman A, Ali A, Njike V, Hom D, Davidi A, Gould-Fogerite S, Milak C, Katz D et al (2012) Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS One 7:e30246
Tj C, Cy L, Yf C, Cj F, Hsu C et al (2015) Acupuncture for treating AI-related arthralgia in breast cancer: a systematic review and meta-analysis. J. Altern. Complement. Med. 21:251–260
Hershman D, Unger J, Vrew K et al (2015) Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of AI-induced musculoskeletal pain: SWOG S0927. J. Clin. Oncol. 33:1910–1917
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, A., De Boer, R., Gan, A. et al. Randomized phase II placebo-controlled study to evaluate the efficacy of topical pure emu oil for joint pain related to adjuvant aromatase inhibitor use in postmenopausal women with early breast cancer: JUST (Joints Under Study). Support Care Cancer 25, 3785–3791 (2017). https://doi.org/10.1007/s00520-017-3810-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3810-9