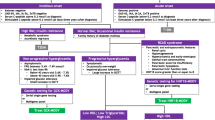
Summary
Maturity onset diabetes of the young (MODY) represents a diabetes type which has an enormous clinical impact. It significantly alters treatment, refines a patient’s prognosis and enables early detection of diabetes in relatives. Nevertheless, when diabetes is manifested the vast majority of MODY patients are not correctly diagnosed, but mostly falsely included among patients with type 1 or type 2 diabetes, in many cases permanently. The aim of this article is to offer a simple and comprehensible guide for recognizing individuals with MODY hidden among adult patients with another type of long-term diabetes and in women with gestational diabetes.
Similar content being viewed by others
References
Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34:1878–84.
Online Mendelian Inheritance in Man, OMIM. Maturity-onset diabetes of the young; MODY. 2016. http://mirror.omim.org/entry/606391. Accessed 15.2019.
Thanabalasingham G, Pal A, Selwood MP, Dudley C, Fisher K, Bingley PJ, et al. Systematic assessment of etiology in adults with a clinical diagnosis of young onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35(6):1206–12.
Brunerova L, Rahelic D, Ceriello A, Broz J. Use of oral antidiabetic drugs in the treatment of maturity-onset diabetes of the young (MODY): a minireview. Diabetes Metab Res Rev. 2018;34(1):e2940.
Shepherd MH, Shields BM, Hudson M, Pearson ER, Hyde C, Ellard S, et al. A UK nationwide prospective study of treatment change in MODY: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin. Diabetologia. 2018;61(12):2520–7. https://doi.org/10.1007/s00125-018-4728-6.
Gardner DS, Tai ES. Clinical features and treatment of maturity onset diabetes of the young (MODY). Diab Meta Syndr Obes Targets Ther. 2012;5:101–8. https://doi.org/10.2147/DMSO.S23353.
Tsai EB, Sherry NA, Palmer JP. Herold KC The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia. 2006;49(2):261–70.
Slingerland A. Monogenic diabetes in children and young adults: challenges for researcher, clinician and patient. Rev Endocr Metab Disord. 2006;7:171–85.
Průhová Š, Dušátková P, Neumann D, Hollay E, Cinek O, Lebl J, et al. Two cases of diabetic ketoacidosis in HNF1A-MODY linked to severe dehydration: is it time to change the diagnostic criteria for MODY? Diabetes Care. 2013;36(9):2573–4.
Horikawa Y, Enya M, Mabe H, Fukushima K, Takubo N, Ohashi M, et al. NEUROD1-deficient diabetes (MODY6): Identification of the first cases in Japanese and the clinical features. Pediatr Diabetes. 2018;19(2):236–42. https://doi.org/10.1111/pedi.12553.
Grzanka M, Matejko B, Szopa M, Kiec-Wilk B, Malecki MT, Klupa T. Assessment of newly proposed clinical criteria to identify HNF1A MODY in patients with an initial diagnosis of type 1 or type 2 diabetes mellitus. Adv Med. 2016; https://doi.org/10.1155/2016/4243784.
Ellard S, Bellanné-Chantelot C, Hattersley AT. European Molecular Genetics Quality Network (EMQN) MODY group. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51(4):546–53.
Shields BM, Shepherd M, Hudson M, McDonald TJ, Colclough K, Peters J, et al. Population-based assessment of a biomarker-based screening pathway to aid diagnosis of Monogenic diabetes in young-onset patients. Diabetes Care. 2017;40(8):1017–25.
Steele AM, Wensley KJ, Ellard S, Murphy R, Shepherd M, Colclough K, et al. Use of HbA1c in the identification of patients with hyperglycaemia caused by a glucokinase mutation: observational case control studies. PLoS ONE. 2013;8:e65326.
Stride A, Shields B, Gill-Carey O, Chakera AJ, Colclough K, Ellard S, et al. Cross sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia. 2014;57(1):54–6.
Pruhova S, Dusatkova P, Kraml PJ, Kulich M, Prochazkova Z, Broz J, et al. Chronic mild hyperglycemia in GCK-MODY patients does not increase carotid intima-media thickness. Int J Endocrinol. 2013; https://doi.org/10.1155/2013/718254.
Chakera AJ, Steele AM, Gloyn AL, Shepherd MH, Shields B, Ellard S, et al. Recognition and management of individuals with hyperglycaemia because of a heterozygous glucokinase mutation. Diabetes Care. 2015;38(7):1383–92.
Stanik J, Dusatkova P, Cinek O, Valentinova L, Huckova M, Skopkova M, et al. De novo mutations of GCK, HNF1A and HNF4A may be more frequent in MODY than previously assumed. Diabetologia. 2014;57(3):480–4.
Steele AM, Shields BM, Shepherd M, Ellard S, Hattersley AT, Pearson ER. Increased all-cause and cardiovascular mortality in monogenic diabetes as a result of mutations in the HNF1A gene. Diabet Med. 2010;27(2):157–61.
Bowman P, Flanagan SE, Edghill EL, Damhuis A, Shepherd MH, Paisey R, et al. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia. 2012;55:123–7.
Naylor R, Knight Johnson A, del Gaudio D. Maturity-onset diabetes of the young overview. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews®. Seattle: University of Washington; 2018. pp. 1993–2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500456/.
Liu L, Nagashima K, Yasuda T, Liu Y, Hu HR, He G, et al. Mutations in KCNJ11 are associated with the development of autosomal dominant, early-onset type 2 diabetes. Diabetologia. 2013;56(12):2609–18.
Sood S, Landreth H, Bustinza J, Chalmers L, Thukaram R. Neonatal diabetes: case report of a 9-week-old presenting diabetic Ketoacidosis Due to an activating ABCC8 gene mutation. Journal of Investigative Medicine High Impact Case Reports. 2017;5(1):2324709617698718.
Pearson ER, Badman MK, Lockwood CR, Clark PM, Ellard S, Bingham C, et al. Contrasting diabetes phenotypes associated with hepatocyte nuclear factor-1alpha and -1beta mutations. Diabetes Care. 2004;27(5):1102–7.
Tonooka N, Tomura H, Takahashi Y, Onigata K, Kikuchi N, Horikawa Y, et al. High frequency of mutations in the HNF-1alpha gene in non-obese patients with diabetes of youth in Japanese and identification of a case of digenic inheritance. Diabetologia. 2002;45(12):1709–12. Dec.
Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol. 2015;11(2):102–12. https://doi.org/10.1038/nrneph.2014.232.
Edghill EL, Bingham C, Slingerland AS, Minton JA, Noordam C, Ellard S, et al. Hepatocyte nuclear factor‑1 beta mutations cause neonatal diabetes and intrauterine growth retardation: support for a critical role of HNF-1beta in human pancreatic development. Diabet Med. 2006;23(12):1301–6.
Weber P, Ambrosova P, Canov P, Weberova D, Kuklinek P, Meluzinova H, et al. GAD antibodies in T1D and LADA—relations to age, BMI, c‑peptide, IA‑2 and HLA-DRB1*03 and DRB1*04 alleles. Adv Gerontol. 2011;24(2):312–8.
Urbanová J, Rypáčková B, Procházková Z, Kučera P, Cerná M, Anděl M, et al. Positivity for islet cell autoantibodies in patients with monogenic diabetes is associated with later diabetes onset and higher HbA1c level. Diabet Med. 2014;31(4):466–71.
Molven A, Ringdal M, Nordbø AM, Raeder H, Støy J, Lipkind GM, et al. Mutation in the insulin gene can cause MODY and antibody-negative type 1 DM. Diabetes. 2008;57:1034–42.
Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present and future. Lancet. 2014;383(9922):1068–83. https://doi.org/10.1016/S0140-6736(13)62154-6.
Stride A, Vaxillaire M, Tuomi T, et al. The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia. 2002;45:427–35. https://doi.org/10.1007/s00125-001-0770-9.
Pearson ER, Liddell WG, Shepherd M, Corrall RJ, Hattersley AT. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1alpha gene mutations: evidence for pharmacogenetics in diabetes. Diabet Med. 2000;17(7):543–5.
McDonald TJ, Shields BM, Lawry J, Owen KR, Gloyn AL, Ellard S, et al. High sensitivity CRP discriminates HNF1A-MODY from other subtypes of diabetes. Diabetes Care. 2011;34(8):1860–2.
Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007;4(4):e118.
Fajans SS, Bell GI, Paz VP, et al. Obesity and hyperinsulinemia in a family with pancreatic agenesis and MODY caused by the IPF1 mutation Pro63fsX60. Transl Res. 2010;156(1):7–14.
Malecki MT, Jhala U, Antonellis A, Fields L, Doria A, Orban T, et al. Mutations in NEUROD1 are associated with the development of type two diabetes. Nat Genet. 1999;23:323–8. https://doi.org/10.1038/15500.
Borowiec M, Liew CW, Thompson R, Boonyasrisawat W, Hu J, Mlynarski WM, et al. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proc Natl Acad Sci U S A. 2009;106:14460–5.
Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, Dina C, Hamid YH, Joly E, et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005;102:4807–12. https://doi.org/10.1073/pnas.0409177102.
Plengvidhya N, Kooptiwut S, Songtawee N, Doi A, Furuta H, Nishi M, et al. PAX4 mutations in Thais with maturity onset diabetes of the young. J Clin Endocrinol Metab. 2007;92:2821–6.
Prundate S, Jungtrakoon P, Marucci A, Ludovico O, Buranasupkajorn P, Mazza T, et al. Loss-of-function mutations in APPL1 in familial diabetes mellitus. Am J Hum Genet. 2015;97:177–85. https://doi.org/10.1016/j.ajhg.2015.05.011.
Raeder H, Johansson S, Holm PI, Haldorsen IS, Mas E, Sbarra V, et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet. 2006;38:54–62.
Gjesing AP, Rui G, Lauenborg J, et al. High prevalence of diabetes-predisposing variants in MODY genes among Danish women with gestational diabetes mellitus. J Endocr Soc. 2017;1(6):681–90. https://doi.org/10.1210/js.2017-00040.
American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetesd 2018. Diabetes Care. 2018;41(Suppl. 1):S13–S27. https://doi.org/10.2337/dc18-S002.
Chakera AJ, Steele AM, Gloyn A, et al. Recognition and management of individuals with hyperglycemia because of heterozygous glucokinase mutations. Diabetes Care. 2015;38:1383–92.
Chakera AJ, Spyer G, Vincent N, Ellard S, Hattersley AT, Dunne FP. The 0.1% of the population with glucokinase monogenic diabetes can be recognized by clinical characteristics in pregnancy: the Atlantic Diabetes in Pregnancy cohort. Diabetes Care. 2014;37:1230–6.
Rudland VL, Hinchcliffe M, Pinner J, Cole S, Mercorella B, et al. Identifying glucokinase monogenic diabetes in a multiethnic gestational diabetes mellitus cohort: new pregnancy screening criteria and utility of HbA1c. Diabetes Care. 2016;39(1):50–2. https://doi.org/10.2337/dc15-1001.
Flack JR, Ross GP, Cheung NW. GCK monogenic diabetes and gestational diabetes: possible diagnosis on clinical grounds. Diabet Med. 2015;32(12):1596–601. https://doi.org/10.1111/dme.12830.
Lachance CH. Practical aspects of monogenic diabetes: a clinical point of view. Canadian Journal of Diabetes. 2016;40(5):368–75.
Bellanné-Chantelot C, Lévy DJ, Carette C, Saint-Martin C, Riveline JP, Larger E, et al. Clinical characteristics and diagnostic criteria of maturity-onset diabetes of the young (MODY) due to molecular anomalies of the HNF1A gene. J Clin Endocrinol Metab. 2011;96:1346–51.
Sung-Hoon K. Maturity-onset diabetes of the young: what do clinicians need to know? Diabetes Metab J. 2015;39(6):468–77.
Acknowledgements
Special thanks to Michael Allen for language editing. The paper was supported by PROGRES Q36 and by MH CZ-DRO (“Kralovske Vinohrady University Hospital —FNKV, 00064173”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Urbanova, L. Brunerova, and J. Broz declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Urbanova, J., Brunerova, L. & Broz, J. How can maturity-onset diabetes of the young be identified among more common diabetes subtypes?. Wien Klin Wochenschr 131, 435–441 (2019). https://doi.org/10.1007/s00508-019-01543-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-019-01543-6