Abstract
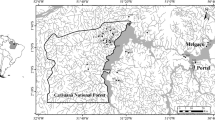
The diversity and abundance of macroinvertebrate shredders were investigated in 52 forested streams (local scale) from nine catchments (regional scale) covering a large area of peninsular Malaysia. A total of 10,642 individuals of aquatic macroinvertebrates were collected, of which 18.22 % were shredders. Biodiversity of shredders was described by alpha (αaverage ), beta (β) and gamma diversity (γ) measures. We found high diversity and abundance of shredders in all catchments, represented by 1,939 individuals (range 6–115 and average per site of 37.29 ± 3.48 SE) from 31 taxa with 2–13 taxa per site (αaverage = 6.98 ± 0.33 SE) and 10–15 taxa per catchment (γ = 13.33 ± 0.55 SE). At the local scale, water temperature, stream width, depth and altitude were correlated significantly with diversity (Adj-R 2 = 0.205). Meanwhile, dissolved oxygen, stream velocity, water temperature, stream width and altitude were correlated to shredder abundance (Adj-R 2 = 0.242). At regional scale, however, water temperature was correlated negatively with β and γ diversity (r 2 = 0.161 and 0.237, respectively) as well as abundance of shredders (r 2 = 0.235). Canopy cover was correlated positively with β diversity (r 2 = 0.378) and abundance (r 2 = 0.266), meanwhile altitude was correlated positively with β (quadratic: r 2 = 0.175), γ diversity (quadratic: r 2 = 0.848) as well as abundance (quadratic: r 2 = 0.299). The present study is considered as the first report describing the biodiversity and abundance of shredders in forested headwater streams across a large spatial scale in peninsular Malaysia. We concluded that water temperature has a negative effect while altitude showed a positive relationship with diversity and abundance of shredders. However, it was difficult to detect an influence of canopy cover on shredder diversity.


Similar content being viewed by others
References
Al-Shami SA, Md Rawi CS, Ahmad A et al (2011) Influence of agricultural, industrial, and anthropogenic stresses on the distribution and diversity of macroinvertebrates in Juru River Basin, Penang, Malaysia. Ecotox Environ Safe 74:1195–1202
Al-Shami SA, Heino J, Che Salmah MR et al (2013a) Drivers of beta diversity of macroinvertebrates communities in tropical forest streams. Freshw Biol. doi:10.1111/fwb.12113
Al-Shami SA, Che Salmah MR, Abu Hassan A et al (2013b) Biodiversity of stream insects in the Malaysian Peninsula: spatial and environmental constraints. Ecol Entoml. doi: 10.1111/een.12013
Angradi TR (1996) Inter-habitat variation in benthic community structure, function, and organic matter storage in 3 Appalachian headwater streams. J N Am Benthol Soc 42–63
Aweng-Eh R, Ismid S, Maketab M et al (2010) Macrobenthic community structure and distribution in the Gunung Berlumut Recreational Forest, Kluang, Johor, Malaysia. Aust J Basic Appl Sci 4(8):3904–3908
Baltz DM, Vondracek B, Brown LR et al (1991) Seasonal changes in microhabitat selection by rainbow trout in a small stream. Trans Am Fish Soc 120:166–176
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Global Ecol Biogeogr 19:134–143
Bernhardt ES, Likens GE, Hall RO Jr et al (2005) Can’t see the forest for the stream? In-stream processing and terrestrial nitrogen exports. BioScience 55:219–230
Boyero L, Ramirez A, Dudgeon D et al (2009) Are tropical streams really different? J N Am Benthol Soc 28:397–403
Boyero L, Pearson RG, Dudgeon D et al (2012) Global patterns of stream detritivore distribution: implications for biodiversity loss in changing climates. Global Ecol Biogeogr 21:134–141
Bunn SE (1988) Processing of leaf litter in a northern Jarrah forest stream. Western Australia: I. Seasonal differences. Hydrobiologia 162:201–210
Che Salmah MR, Al-Shami SA, Madrus MR et al (2013a) Local effects of forest fragmentation on diversity of aquatic insects in tropical forest streams: implications for biological conservation. Aquat Ecol. doi:10.1007/s10452-012-9426-8
Che Salmah MR, Al-Shami SA, Madrus MR et al (2013b) Biological and ecological diversity of aquatic macroinvertebrates in response to hydrological and physicochemical parameters in tropical forest streams of Gunung Tebu, Malaysia: implications for ecohydrological assessment. Ecohydrology. doi:10.1002/eco.1368
Cheshire K, Boyero L, Pearson RG et al (2005) Food webs in tropical Australian streams: shredders are not scarce. Freshwater Biol 50:748–769
Clarke A, Mac Nally R, Bond N et al (2008) Macroinvertebrate diversity in headwater streams: a review. Freshw Biol 53:1707–1721
Cummins KW, Klug MJ (1979) Feeding ecology of stream invertebrates. Annu Rev Ecol Syst 10:147–172
Dangles OJ, Guérold FA (2000) Structural and functional responses of benthic macroinvertebrates to acid precipitation in two forested headwater streams (Vosges Mountains, northeastern France). Hydrobiologia 418:25–31
Davies P, Cook L, McIntosh P et al (2005) Changes in stream biota along a gradient of logging disturbance, 15 years after logging at Ben Nevis, Tasmania. Forest Ecol Manag 219:132–148
R Development Core Team (2010) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://www.R-project.org
Dobson M, Magana A, Mathooko JM et al (2002) Detritivores in Kenyan highland streams: more evidence for the paucity of shredders in the tropics? Freshw Biol 47:909–919
Douglas I, Spencer T, Greer T et al (1992) The impact of selective commercial logging on stream hydrology, chemistry and sediment loads in the Ulu Segama Rain-Forest, Sabah, Malaysia. Philos Trans R Soc B 335:397–406
Dudgeon D (1994) The influence of riparian vegetation on macroinvertebrate community structure and functional organization in six New Guinea streams. Hydrobiologia 294:65–85
Dudgeon D (2006) The impacts of human disturbance on stream benthic invertebrates and their drift in North Sulawesi, Indonesia. Freshw Biol 51:1710–1729
Dudgeon D (2008) Tropical streams ecology. Elsevier, New York
Dudgeon A, Bretschko G (1996) Allochthonous inputs and land-water interactions in seasonal streams: tropical Asia and temperate Europe. In: Schiemer F, Boland KT (eds) Perspectives in Tropical Limnology. SPB, Amsterdam, pp 161–179
Elliott JM (1971) Some methods for the statistical analysis of samples of benthic invertebrates. Freshwater Biological Association, Ambleside, UK
Gonçalves JF Jr, Graça MAS, Callisto M et al (2006) Leaf-litter breakdown in 3 streams in temperate, Mediterranean, and tropical Cerrado climates. J N Am Benthol Soc 25:344–355
Greathouse EA, Pringle CM (2006) Does the river continuum concept apply on a tropical island? Longitudinal variation in a Puerto Rican stream. Can J Fish Aquat Sci 63:134–152
Gronroos M, Heino J (2012) Species richness at the guild level: effects of species pool and local environmental conditions on stream macroinvertebrate communities. J Anim Ecol 81:679–691
Hall RO Jr, Likens GE, Malcom HM et al (2001) Trophic basis of invertebrate production in 2 streams at the Hubbard Brook Experimental Forest. J N Am Benthol Soc 20:432–447
Hawkins CP, Sedell JR (1981) Longitudinal and seasonal changes in functional organization of macroinvertebrate communities in four Oregon streams. Ecology: 387–397
Hector A, Fowler D, Nussbaum R et al (2011) The future of South East Asian rainforests in a changing landscape and climate introduction. Philos Trans R Soc B 366:3165–3167
Heino J (2005) Functional biodiversity of macroinvertebrate assemblages along major ecological gradients of boreal headwater streams. Freshw Biol 50:1578–1587
Heino J (2009) Biodiversity of aquatic insects: spatial gradients and environmental correlates of assemblage-level measures at large scales. Freshw Rev 2:1–29
Heino J, Soininen J (2007) Are higher taxa adequate surrogates for species-level assemblage patterns and species richness in stream organisms? Biol Conserv 137:78–89
Hynes HBN (1970) The ecology of running waters. Liverpool University Press, Liverpool
Irons JG III, Oswood MW, Stout R et al (1994) Latitudinal patterns in leaf litter breakdown: is temperature really important? Freshw Biol 32:401–411
Jacobsen D, Schultz R, Encalada A et al (1997) Structure and diversity of stream invertebrate assemblages: the influence of temperature with altitude and latitude. Freshw Biol 38:247–261
Jiang X, Xiong J, Xie Z et al (2011) Longitudinal patterns of macroinvertebrate functional feeding groups in a Chinese river system: a test for river continuum concept (RCC). Quat Int 244:289–295
Jung SW, Nguyen VV, Nguyen QH et al (2008) Aquatic insect faunas and communities of a mountain stream in Sapa Highland, northern Vietnam. Limnology 9:219–229
Lecerf A, Usseglio-Polatera P, Charcosset JY et al (2006) Assessment of functional integrity of eutrophic streams using litter breakdown and benthic macroinvertebrates. Arch Hydrobiol 165:105–126
Leroy CJ, Marks JC (2006) Litter quality, stream characteristics and litter diversity influence decomposition rates and macroinvertebrates. Freshw Biol 51:605–617
Li AOY, Dudgeon D (2009) Shredders: species richness, abundance, and role in litter breakdown in tropical Hong Kong streams. J N Am Benthol Soc 28:167–180
Magurran AE (2004) Measuring biological diversity. Blackwell, Oxford
Mathuriau C, Chauvet E (2002) Breakdown of leaf litter in a neotropical stream. J N Am Benthol Soc 21:384–396
Merritt R, Cummins K (1996) Trophic relations of macroinvertebrates. Methods in stream ecology. Hauer FR, Lamberti GA (eds) Academic, San Diego, pp 453–474
Morse JC, Yang L, Tian L et al (1994) Aquatic insects of China useful for monitoring water quality. Hohai University Press
Murphy JF, Giller PS (2000) Seasonal dynamics of macroinvertebrate assemblages in the benthos and associated with detritus packs in two low–order streams with different riparian vegetation. Freshw Biol 43:617–631
Nicola GG, Almodóvar A, Elvira B et al (2010) Effects of environmental factors and predation on benthic communities in headwater streams. Aquat Sci Res Across Boundaries 72:419–429
Palmer MW (1990) The estimation of species richness by extrapolation. Ecology 71:1195–1198
Pearson R, Tobin R, Smith R et al (1989) Standing crop and processing of rainforest litter in a tropical Australian stream. Arch Hydrobiol 115:481–498
Platts WS, Megahan WF, Minshall GW (1983) Methods for evaluating stream, riparian, and biotic conditions. Intermountain Forest and Range Experiment Station, Ogden, UT
Sodhi NS, Brook BW (2006) Southeast Asian biodiversity in crisis. Cambridge University Press, Cambridge, UK
Stirling G, Wilsey B (2001) Empirical relationships between species richness, evenness, and proportional diversity. Am Nat 158:286–299
Stone MK, Wallace JB (1998) Long–term recovery of a mountain stream from clear–cut logging: the effects of forest succession on benthic invertebrate community structure. Freshw Biol 39:151–169
Suhaila AH (2011) Abundance and diversity of Ephemeroptera, Plecoptera and Trichoptera (EPT) in relation to environmental quality of upstream rivers in Kedah, Malaysia. Unpublished PhD thesis, Universiti Sains Malaysia, Malaysia
Suren AM (1994) Macroinvertebrate communities of streams in western Nepal: effects of altitude and land use. Freshw Biol 32:323–336
Tiegs S, Peter F, Robinson C et al (2008) Leaf decomposition and invertebrate colonization responses to manipulated litter quantity in streams. J N Am Benthol Soc 27:321–331
Tomanova S, Tedesco PA, Campero M et al (2007) Longitudinal and altitudinal changes of macroinvertebrate functional feeding groups in neotropical streams: a test of the river continuum concept. Fund Appl Limnol 170:233–241
Touma BR, Encalada AC, Fornells NP et al (2009) Leaf litter dynamics and its use by invertebrates in a high–altitude tropical Andean stream. Int Rev Hydrobiol 94:357–371
Vannote RL, Minshall GW, Cummins KW et al (1980) River continuum concept. Can J Fish Aquat Sci 37:130–137
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, Berlin
Wallace JB, Eggert SL, Meyer JL et al (1997) Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277:102–104
Wantzen KM, Wagner R, Suetfeld R et al (2002) How do plant-herbivore interactions of trees influence coarse detritus processing by shredders in aquatic ecosystems of different latitudes? Int Ver Verh Int Ver Theor Angew Limnol Ver 28:815–821
Webster J, Benfield E (1986) Vascular plant breakdown in freshwater ecosystems. Annu Rev Ecol Syst 17:567–594
Yule CM (1996) Trophic relationships and food webs of the benthic invertebrate fauna of two aseasonal tropical streams on Bougainville Island, Papua New Guinea. J Trop Ecol 12:517–534
Yule CM, Gomez LN (2009) Leaf litter decomposition in a tropical peat swamp forest in Peninsular Malaysia. Wetl Ecol Manag 17:231–241
Yule C, Yong H (2004) Freshwater Invertebrates of the Malaysian Region. Akademi Sains Malaysia, Kuala Lumpur
Yule CM, Leong MY, Liew KC et al (2009) Shredders in Malaysia: abundance and richness are higher in cool upland tropical streams. J N Am Benthol Soc 28:404–415
Acknowledgments
We would like to thank Hazdri Abdullah, Shukri, Hamzah, Siti Khatijah, Tahir, Wan Zaki , Kalimuthu for their tireless help in the field. To many others who helped us directly or indirectly during this study, we are deemed indebted. We are grateful to the Dean, School of Biological Sciences, Universiti Sains Malaysia in Penang for providing field and laboratory facilities. We also thank Forest Research Institute Malaysia counterparts headed by Dr Christine Fletcher and Dr Abdul Rahman Kassim, for financial support, help and understanding. The Conservation of Biodiversity (CBioD) Project is a national project executed by the Ministry of Natural Resources and the Environment, and implemented by the Forest Research Institute Malaysia. The CBioD Project is co-funded by the UNDP-GEF (MAL/04/G3) and ITTO [PD 165 02 Rev.3 (F)]. Key partners to the CBioD Project are: Perak ITC S/B, Perak SEDC, Forestry Headquarters and State Forestry Departments of Peninsular Malaysia. The Project is a joint effort with the University of Miami, Duke University and Harvard University. Thanks to three anonymous reviewers for their constructive comments and suggestions, which all improved this paper significantly.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLS 40 kb)
Rights and permissions
About this article
Cite this article
Che Salmah, M.R., Al-Shami, S.A., Abu Hassan, A. et al. Distribution of detritivores in tropical forest streams of peninsular Malaysia: role of temperature, canopy cover and altitude variability. Int J Biometeorol 58, 679–690 (2014). https://doi.org/10.1007/s00484-013-0648-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-013-0648-9