Abstract
Key message
Physicochemical conditions present during the initiation of somatic embryogenesis in Pinus radiata determine the rate of embryogenic cell lines generated as well as the final number of somatic embryos.
Abstract
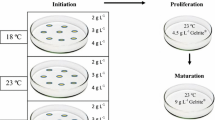
The effect of physical and chemical conditions during the initiation and proliferation stages of somatic embryogenesis in radiata pine was investigated. The first objective was to assess if different temperatures and/or water availability during the initiation or proliferation of embryogenic tissue impacted the success rate during each stage of somatic embryogenesis. The second objective was to determine what stage has a greater influence on subsequent stages of development. Some treatments (18 °C, 4 g L−1 gellan gum) resulted in a higher percentage of initiation whereas others (28 °C, 2 g L−1 gellan gum) gave rise to lower initiation. Our results indicated that initiation at different temperatures affected the subsequent stages of somatic embryogenesis. Thus, these embryogenic tissues were influenced by the environmental conditions present at initiation. We were able to successfully regenerate somatic embryos from cell lines initiated under all the environmental conditions tested.














Similar content being viewed by others
Abbreviations
- ECLs:
-
Embryogenic cell lines
- EDM:
-
Embryo development medium
- EM:
-
Embryonal mass
- EMs:
-
Embryonal masses
- SE:
-
Somatic embryogenesis
- se:
-
Somatic embryos
References
Aitken-Christie J, Singh AP, Davies H (1988) Multiplication of meristematic tissue: a new tissue culture system for radiata pine. In: Hanover JW, Keathley DE (eds) Genetic manipulation of woody plants. Plenum Publishing Corp, New York, pp 413–432
Ascough GD, Fennell CW (2004) The regulation of plant growth and development in liquid culture. S Afr J Bot 70:181–190
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188
Beruto M, Beruto D, Debergh P (1999) Influence of agar on in vitro cultures. I. Physiochemical properties of agar and agar gelled media. In Vitro Cell Dev Biol 35:86–93
Bonga JM, Klimaszewska K, Von Aderkas P (2010) Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tissue Organ Culture 100:241–254
Choudhury H, Kumaria S, Tandon P (2008) Induction and maturation of somatic embryos from intact megagametophyte explants in Khasi pine (Pinus kesiya Royle ex. Gord.). Curr Sci 95:1433–1438
Fehér A (2015) Somatic embryogenesis—stress-induced remodeling of plant cell fate. Biochim Biophys Acta 1849:385–402
Hargreaves CL, Grace LJ, Holden DG (2002) Nurse culture for efficient recovery of cryopreserved Pinus radiata D. Don embryogenic cell lines. Plant Cell Rep 21:40–45
Hargreaves CL, Reeves CB, Find JI, Gough K, Josekutty P, Skudder DB, Van der Maas SA, Sigley MR, Menzies MI, Low CB, Mullin TJ (2009) Improving initiation, genotype capture, and family representation in somatic embryogenesis of Pinus radiata by a combination of zygotic embryo maturity, media, and explant preparation. Can J Forest Res 39:1566–1574
Harry IS, Thorpe TA (1991) Somatic embryogenesis and plant regeneration from mature zygotic embryos of red spruce. Bot Gaz 152:446–452
Ikeda-Iwai M, Umehara M, Satoh S, Kamada H (2003) Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J 34:107–114
Jördy M (2004) Seasonal variation of organogenetic activity and reserves allocation in the shoot apex of Pinus pinaster Ait. Ann Bot Lond 93:25–37
Klimaszewska K, Bernier-Cardou M, Cyr DR, Sutton BCS (2000) Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. In Vitro Cell Dev Biol Plant 36:279–286
Klimaszewska K, Park YS, Overton C, MacEacheron I, Bonga JM (2001) Optimized somatic embryogenesis in Pinus strobus L. In Vitro Cell Dev Biol Plant 37:392–399
Klimaszewska K, Trontin JF, Becwar MR, Devillard C, Park YS, Lelu-Walter MA (2007) Recent progress in somatic embryogenesis of four Pinus spp. Tree Forest Sci Biotechnol 1:11–25
Kvaalen H, Johnsen Ø (2008) Timing of bud set in Picea abies is regulated by a memory of temperature during zygotic and somatic embryogenesis. New Phytol 177:49–59
Lelu-Walter MA, Bernier-Cardou M, Klimaszewska K (2008) Clonal plant production from self- and cross-pollinated seed families of Pinus sylvestris (L.) through somatic embryogenesis. Plant Cell Tissue Organ Culture 92:31–45
Li B, Wolyn D (1996) Temperature and genotype affect asparagus somatic embryogenesis. In Vitro Cell Dev Biol Plant 32:136–139
Li XY, Huang FH, Murphy JB, Gbur EE (1998) Polyethylene glycol and maltose enhance somatic embryo maturation in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol Plant 34:22–26
McCulloch CE, Searle SR (2001) Generalized, linear and mixed models. Wiley-Interscience, New York
Montalbán IA, De Diego N, Moncaleán P (2010) Bottlenecks in Pinus radiata somatic embryogenesis: improving maturation and germination. Trees Struct Funct 24:1061–1071
Montalbán IA, De Diego N, Aguirre-Igartua E, Setién A, Moncaleán P (2011) A combined pathway of somatic embryogenesis and organogenesis to regenerate radiata pine plants. Plant Biotechnol Rep 5:177–186
Montalbán IA, De Diego N, Moncaleán P (2012) Enhancing initiation and proliferation in radiata pine (Pinus radiata D. Don) somatic embryogenesis through seed family screening, zygotic embryo staging and media adjustments. Acta Physiol Plant 34:451–460
Montalbán IA, García-Mendiguren O, Goicoa T, Ugarte MD, Moncaleán P (2015) Cold storage of initial plant material affects positively somatic embryogenesis in Pinus radiata. New Forest 46:157–165
Morel A, Teyssier C, Trontin J-F, Eliášová K, Pešek B, Beaufour M, Morabito D, Boizot N, Le Metté C, Belal-Bessai L, Reymond I, Harvengt L, Cadene M, Corbineau F, Vágner M, Label P, Lelu-Walter M-A (2014) Early molecular events involved in Pinus pinaster Ait. somatic embryo development under reduced water availability: transcriptomic and proteomic analyses. Physiol Plant 152:184–201
Neilson KA, Gammulla CG, Mirzaei M, Imin N, Haynes PA (2010) Proteomic analysis of temperature stress in plants. Proteomics 10:828–845
Park YS (2002) Implementation of conifer somatic embryogenesis in clonal forestry: technical requirements and deployment considerations. Ann Forest Sci 59:651–656
Park YS, Barrett JD, Bonga JM (1998) Application of somatic embryogenesis in high-value clonal forestry: deployment, genetic control, and stability of cryopreserved clones. In Vitro Cell Dev Biol Plant 34:231–239
Park YS, Lelu-Walter MA, Harvengt L, Trontin JF, MacEacheron I, Klimaszewska K, Bonga JM (2006) Initiation of somatic embryogenesis in Pinus banksiana, P. strobus, P. pinaster and P. sylvestris at three laboratories in Canada and France. Plant Cell Tissue Organ Culture 86:87–101
Pullman GS, Johnson S (2002) Somatic embryogenesis in loblolly pine (Pinus taeda L.): improving culture initiation rates. Ann Forest Sci 59:663–668
Pullmann G, Skryabina A (2007) Liquid medium and liquid overlays improve embryogenic tissue initiation in conifers. Plant Cell Rep 26:873–887
Quoirin M, Lepoivre P (1977) Études des milieux adaptés aux cultures in vitro de Prunus. Acta Hortic 78:437–442
Salajova T, Salaj J (2005) Somatic embryogenesis in Pinus nigra: embryogenic tissue initiation, maturation and regeneration ability of established cell lines. Biol Plant 49:333–339
Smith DR (1997) The role of in vitro methods in pine plantation establishment: the lesson from New Zealand. Plant Tissue Cult Biotechnol 3:63–73
Stasolla C, Yeung EC (2003) Recent advances in conifer somatic embryogenesis: improving somatic embryo quality. Plant Cell Tissue Organ Culture 74:15–35
Teyssier C, Grondin C, Bonhomme L, Lomenech AM, Vallance M, Morabito D, Label P, Lelu-Walter MA (2011) Increased gelling agent concentration promotes somatic embryo maturation in hybrid larch (Larix × eurolepsis): a 2-DE proteomic analysis. Physiol Plantarum 141:152–165
Von Aderkas P, Bonga JM (2000) Influencing micropropagation and somatic embryogenesis in mature trees by manipulation of phase change, stress and culture environment. Tree Physiol 20:921–928
Walter C (2004) Genetic engineering in conifer forestry: technical and social considerations. In Vitro Cell Dev Biol Plant 40:434–441
Walter C, Smith DR, Connett MB, Grace L, White DWR (1994) A biolistic approach for the transfer and expression of a gusA reporter gene in embryogenic cultures of Pinus radiata. Plant Cell Rep 14:69–74
Zhang CX, Li Q, Kong L (2007) Induction, development and maturation of somatic embryos in Bunge’s pine (Pinus bungeana Zucc. ex Endl.). Plant Cell Tissue Organ Culture 91:273–280
Acknowledgments
This research was funded by MINECO (Spanish Government) project (AGL2013-4700-C4-2R) and DECO (Basque Government) who awarded Olatz García-Mendiguren with a Ph.D. scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Additional information
Communicated by K. Klimaszewska.
I. A. Montalbán and P. Moncaleán contributed equally.
Rights and permissions
About this article
Cite this article
García-Mendiguren, O., Montalbán, I.A., Goicoa, T. et al. Environmental conditions at the initial stages of Pinus radiata somatic embryogenesis affect the production of somatic embryos. Trees 30, 949–958 (2016). https://doi.org/10.1007/s00468-015-1336-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1336-7