Abstract
Background
Infusion reaction (IR) is defined as an adverse event within 24 h after monoclonal antibody infusion. In non-Hodgkin lymphoma, IR incidence following rituximab treatment is high (77–80%), but there are no data in complicated nephrotic syndrome.
Methods
Records of rituximab infusions in patients with complicated nephrotic syndrome between February 2006 and December 2014 at the National Center for Child Health and Development were reviewed. Rituximab was administered at doses of 375 mg/m2. The severity of IR was evaluated using the Common Terminology Criteria for Adverse Events ver. 4.0.
Results
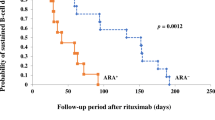
For 309 rituximab infusions in 159 patients (male, 110; median age, 12 years), IR was observed in 165 infusions (53.4%). Respiratory symptoms were most common (66% of all events). Ninety-five percent of the IR was observed within 3 h after rituximab infusion initiation. Sixty-eight percent of the events were classified as grade 1 and others classified as grade 2. Only 18% required medical intervention. CD20 cell count in patients with IR was significantly higher than in patients without IR. Incidence of IR was similar in subsequent rituximab treatment after B-cell recovery. Patients who experienced IR at first rituximab treatment were more likely to experience recurrent IR with subsequent treatments compared to those not having IR at initial treatment (odds ratio 3.64; p < 0.001).
Conclusions
In patients with complicated nephrotic syndrome, respiratory symptoms were the major type of IR, mostly observed within 3 h of infusion. Incidence of IR was lower and its severity milder in patients with complicated nephrotic syndrome than those with lymphoma.


Similar content being viewed by others
References
Vogel WH (2010) Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs 14:E10–E21
Winkler U, Jensen M, Manzke O, Schulz H, Diehl V, Engert A (1999) Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 94:2217–2224
Bienvenu J, Chvetzoff R, Salles G, Balter C, Tilly H, Herbrecht R, Morel P, Lederlin P, Solal-Celigny P, Audhuy B, Christian B, Gabarre J, Casasnovas O, Marit G, Sebban C, Coiffier B, Groupe d’Etude des Lymphomes de l’Adulte (2001) Tumor necrosis factor alpha release is a major biological event associated with rituximab treatment. Hematol J 2:378–384
Byrd JC, Murphy T, Howard RS, Lucas MS, Goodrich A, Park K, Pearson M, Waselenko JK, Ling G, Grever MR, Grillo-Lopez AJ, Rosenberg J, Kunkel L, Flinn IW (2001) Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol 19:2153–2164
Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, Levy R (1994) Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood 84:2457–2466
Maloney DG, Grillo-López AJ, Bodkin DJ, White CA, Liles TM, Royston I, Varns C, Rosenberg J, Levy R (1997) IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol 15:3266–3274
Maloney DG, Grillo-López AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, Wey K, Royston I, Davis T, Levy R (1997) IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90:2188–2195
McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK (1998) Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825–2833
Davis TA, White CA, Grillo-López AJ, Velásquez WS, Link B, Maloney DG, Dillman RO, Williams ME, Mohrbacher A, Weaver R, Dowden S, Levy R (1999) Single-agent monoclonal antibody efficacy in bulky non-Hodgkin’s lymphoma: results of a phase II trial of rituximab. J Clin Oncol 17:1851–1857
Piro LD, White CA, Grillo-López AJ, Janakiraman N, Saven A, Beck TM, Varns C, Shuey S, Czuczman M, Lynch JW, Kolitz JE, Jain V (1999) Extended Rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol 10:655–661
Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL, Rosenberg J, Levy R (2000) Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol 18:3135–3143
Lenz HJ (2007) Management and preparedness for infusion and hypersensitivity reactions. Oncologist 12:601–609
Schwartzberg LS, Stepanski EJ, Fortner BV, Houts AC (2008) Retrospective chart review of severe infusion reactions with rituximab, cetuximab, and bevacizumab in community oncology practices: assessment of clinical consequences. Support Care Cancer 16:393–398
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235–242
Kunkel L, Wong A, Maneatis T, Nickas J, Brown T, Grillo-López A, Benyunes M, Grobman B, Dillman RO (2000) Optimizing the use of rituximab for treatment of B-cell non-Hodgkin's lymphoma: a benefit-risk update. Semin Oncol 27(6 Suppl 12):53–61
Chung CH (2008) Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist 13:725–732
Ishikura K, Matsumoto S, Sako M, Tsuruga K, Nakanishi K, Kamei K, Saito H, Fujinaga S, Hamasaki Y, Chikamoto H, Ohtsuka Y, Komatsu Y, Ohta T, Nagai T, Kaito H, Kondo S, Ikezumi Y, Tanaka S, Kaku Y, Iijima K, Japanese Society for Pediatric Nephrology (2015) Clinical practice guideline for pediatric idiopathic nephrotic syndrome 2013: medical therapy. Clin Exp Nephrol 19:6–33
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y, Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group (2014) Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384:1273–1281
Legeay C, Bittencourt H, Haddad E, Spiesser-Robelet L, Thépot-Seegers V, Therrien R (2017) A retrospective study on infusion-related reactions to rituximab in a heterogeneous pediatric population. J Pediatr Pharmacol Ther 22:369–374
Henriquez KM, Hayney MS, Xie Y, Zhang Z, Barrett B (2015) Association of interleukin-8 and neutrophils with nasal symptom severity during acute respiratory infection. J Med Virol 87:330–337
Pitrez PM, Brennan S, Sly PD (2005) Inflammatory profile in nasal secretions of infants hospitalized with acute lower airway tract infections. Respirology 10:365–370
Kamei K, Takahashi M, Fuyama M, Saida K, Machida H, Sato M, Ogura M, Ito S (2015) Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: case series and review of literature. Nephrol Dial Transplant 30:91–96
Sato M, Ito S, Ogura M, Kamei K, Miyairi I, Miyata I, Higuchi M, Matsuoka K (2013) Atypical Pneumocystis jiroveci pneumonia with multiple nodular granulomas after rituximab for refractory nephrotic syndrome. Pediatr Nephrol 28:145–149
Chaumais MC, Garnier A, Chalard F, Peuchmaur M, Dauger S, Jacqz-Agrain E, Deschênes G (2009) Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol 24:1753–1755
Keir GJ, Maher TM, Ming D, Abdullah R, de Lauretis A, Wickremasinghe M, Nicholson AG, Hansell DM, Wells AU, Renzoni EA (2014) Rituximab in severe, treatment-refractory interstitial lung disease. Respirology 19:353–359
Sano Y, Nakano Y, Omoto M, Takao M, Ikeda E, Oga A, Nakamichi K, Saijo M, Maoka T, Sano H, Kawai M, Kanda T (2015) Rituximab-associated progressive multifocal leukoencephalopathy derived from non-Hodgkin lymphoma: neuropathological findings and results of mefloquine treatment. Intern Med 54:965–970
Sokol J, Lisá L, Zeleňáková J, Balhárek T, Plameňová I, Staško J, Kubisz P (2017) Rituximab-associated progressive multifocal leukoencephalopathy. Vnitr Lek 63:60–64
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KK has received lecture fees from the Chugai Pharmaceutical Co., Ltd. SI has received lecture fees and/or grants from the Chugai Pharmaceutical Co., Ltd.; Zenyaku Kogyo Co., Ltd.; Asahi Kasei Pharma; Novartis Pharma K.K.; and Astellas Pharma Inc. KI has received lecture fees from the Asahi Kasei Pharma; Chugai Pharmaceutical Co., Ltd.; Zenyaku Kogyo Co., Ltd.; and Novartis Pharma K.K. and a consultant fee from Ono Pharmaceutical Co., Ltd.
Ethical approval
The design and execution of this study were in accordance with the ethical standards of the Declaration of Helsinki. The protocol was approved by the Ethics Committee of the National Center for Child Health and Development (no. 1505).
Informed consent
For this type of study, formal informed consent is not required.
Rights and permissions
About this article
Cite this article
Kamei, K., Ogura, M., Sato, M. et al. Infusion reactions associated with rituximab treatment for childhood-onset complicated nephrotic syndrome. Pediatr Nephrol 33, 1013–1018 (2018). https://doi.org/10.1007/s00467-018-3900-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-3900-z