Abstract
Background
Darbepoetin alfa is a commonly prescribed erythropoiesis-stimulating agent (ESA) for correcting anemia in pediatric chronic kidney disease (CKD) patients. However, little information exists on its use in ESA-naïve patients. This study evaluated the efficacy and safety of darbepoetin alfa in pediatric patients initiating ESA therapy.
Methods
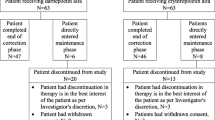
One-hundred sixteen pediatric ESA-naïve subjects (aged 1–18 years) with CKD stages 3–5D and hemoglobin (Hb) <10 g/dl from 43 centers in the US, Europe, and Mexico were randomized by age (three groups) and dialysis status (yes vs. no) to receive darbepoetin alfa once weekly (QW) or every 2 weeks (Q2W) subcutaneously (not on dialysis and peritoneal dialysis subjects) and intravenously (hemodialysis subjects). The drug was titrated to achieve Hb levels of 10.0–12.0 g/dl over 25 weeks. Patient- and parent-reported health-related outcomes were measured by the Pediatric Quality of Life Inventory (PedsQL™) in children ≥2 years.
Results
In both groups, mean Hb concentrations increased to ≥11.0 g/dl over the first 3 months of treatment and remained stable within the 10.0–12.0 g/dl target range. The median time to achieve hemoglobin ≥10 g/dl was slightly longer for subjects <12 years (QW and Q2W, both 28 days) vs. those ≥12 years (23 and 22 days, respectively). Adverse event profiles were similar between groups, with QW, four (7%) and Q2W, five (9%). PedsQL™ scores showed modest increases.
Conclusions
Darbepoetin alfa can be safely administered either QW or Q2W to ESA-naïve pediatric patients with CKD-related anemia to achieve Hb targets of 10.0–12.0 g/dl.




Similar content being viewed by others
References
United States Renal Data System (2012) USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
Mitsnefes MM, Laskin BL, Dahhou M, Zhang X, Foster BJ (2013) Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990-2010. JAMA 309:1921–1929
Bahlmann J, Schöter KH, Scigalla P, Gurland HJ, Hilfenhaus M, Koch KM, Muthny FA, Neumayer HH, Pommer W, Quelhorst E, Sieberth HG, Weber U (1991) Morbidity and mortality in hemodialysis patients with and without erythropoietin treatment: a controlled study. Contrib Nephrol 88:90–106
Bárány P, Pettersson E, Konarski-Svensson JK (1993) Long-term effects on quality of life in haemodialysis patients of correction of anaemia with erythropoietin. Nephrol Dial Transplant 8:426–432
Evans RW, Rader B, Manninen DL (1990) The quality of life of hemodialysis recipients treated with recombinant human erythropoietin. Cooperative multicenter EPO clinical trial group. JAMA 263:825–830
Foley RN, Parfrey PS, Morgan J, Barré PE, Campbell P, Cartier P, Coyle D, Fine A, Handa P, Kingma I, Lau CY, Levin A, Mendelssohn D, Muirhead N, Murphy B, Plante RK, Posen G, Wells GA (2000) Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int 58:1325–1335
Furuland H, Linde T, Ahlmén J, Christensson A, Strömbom U, Danielson BG (2003) A randomized controlled trial of haemoglobin normalization with epoetin alfa in pre-dialysis and dialysis patients. Nephrol Dial Transplant 18:353–361
Gerson A, Hwang W, Fiorenza J, Barth K, Kaskel F, Weiss L, Zelikovsky N, Fivush B, Furth S (2004) Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis 44:1017–1023
Canadian Erythropoietin Study Group (1990) Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. BMJ 300:573–578
Marsh JT, Brown WS, Wolcott D, Carr CR, Harper R, Schweitzer SV, Nissenson AR (1991) rHuEPO treatment improves brain and cognitive function of anemic dialysis patients. Kidney Int 39:155–163
Muirhead N, Laupacis A, Wong C (1992) Erythropoietin for anaemia in haemodialysis patients: results of a maintenance study (the Canadian erythropoietin study group). Nephrol Dial Transplant 7:811–816
Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B, for the CKiD Study Group (2010) Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol 21:137–144
Atkinson MA, Lestz RM, Fivush BA, Silverstein DM (2011) Comparative clinical outcomes between pediatric and young adult dialysis patients. Pediatr Nephrol 26:2219
Klang B, Björvell H, Clyne N (1996) Quality of life in predialytic uremic patients. Qual Life Res 5:109–116
Pickett JL, Theberge DC, Brown WS, Schweitzer SU, Nissenson AR (1999) Normalizing hematocrit in dialysis patients improves brain function. Am J Kidney Dis 33:1122–1130
Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA (1998) The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339:584–590
Eschbach JW (1994) Erythropoietin: the promise and the facts. Kidney Int Suppl 44:S70–S76
Provenzano R, Garcia-Mayol L, Suchinda P, Von Hartitzsch B, Woollen SB, Zabaneh R, Fink JC, POWER Study Group (2004) Once-weekly epoetin alfa for treating the anemia of chronic kidney disease. Clin Nephrol 61:392–405
Valderrábano F, Hörl WH, Macdougall IC, Rossert J, Rutkowski B, Wauters JP (2003) PRE-dialysis survey on anaemia management. Nephrol Dial Transplant 18:89–100
North American Pediatric Renal Trials and Collaborative Studies (2014) Annual Transplant Report. http://spitfire.emmes.com/study/ped/annlrept/annualrept2014.pdf
Elliott S, Lorenzini T, Asher S, Aoki K, Brankow D, Buck L, Busse L, Chang D, Fuller J, Grant J, Hernday N, Hokum M, Hu S, Knudten A, Levin N, Komorowski R, Martin F, Navarro R, Osslund T, Rogers G, Rogers N, Trail G, Egrie J (2003) Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol 21:414–421
Egrie JC, Dwyer E, Browne JK, Hitz A, Lykos MA (2003) Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol 31:290–299
Lerner G, Kale AS, Warady BA, Jabs K, Bunchman TE, Heatherington A, Olson K, Messer-Mann L, Maroni BJ (2002) Pharmacokinetics of darbepoetin alfa in pediatric patients with chronic kidney disease. Pediatr Nephrol 17:933–937
Warady BA, Arar MY, Lerner G, Nakanishi AM, Stehman-Breen C (2006) Darbepoetin alfa for the treatment of anemia in pediatric patients with chronic kidney disease. Pediatr Nephrol 21:1144–1152
Schaefer F, Hoppe B, Jungraithmayr T, Klaus G, Pape L, Farouk M, Addison J, Manamley N, Vondrak K (2016) Safety and usage of darbepoetin alfa in children with chronic kidney disease: prospective registry study. Pediatr Nephrol 31:443–453
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Varni JW, Burwinkle TM, Seid M, Skarr D (2003) The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 3:329–341
Doshi S, Chow A, Pérez Ruixo JJ (2010) Exposure-response modeling of darbepoetin alfa in anemic patients with chronic kidney disease not receiving dialysis. J Clin Pharmacol 50:75S–90S
Atkinson MA, Martz K, Warady BA, Neu AM (2010) Risk for anemia in pediatric chronic kidney disease patients: a report of NAPRTCS. Pediatr Nephrol 25:1699–1706
André J-L, Deschênes G, Boudailliez B, Broux F, Fischbach M, Gagnadoux M-F, Horen B, Lahoche-Manucci A, Macher M-A, Roussel B, Tsimaratos M, Loirat C (2007) Darbepoetin, effective treatment of anaemia in paediatric patients with chronic renal failure. Pediatr Nephrol 22:708–714
Geary DF, Keating LE, Vigneux A, Stephens D, Hébert D, Harvey EA (2005) Darbepoetin alfa (Aranesp™) in children with chronic renal failure. Kidney Int 68:1759–1765
Hattori M, Matsunaga A, Akioka Y, Fujinaga S, Nagai T, Uemura O, Nakakura H, Ashida A, Kamei K, Ito S, Yamada T, Goto Y, Ohta T, Hisano M, Komatsu Y, Itami N (2013) Darbepoetin alfa for the treatment of anemia in children undergoing peritoneal dialysis: a multicenter prospective study in Japan. Clin Exp Nephrol 17:582–588
Warady BA, Silverstein DM (2014) Management of anemia with erythropoietic-stimulating agents in children with chronic kidney disease. Pediatr Nephrol 29:1493–1505
Gerson AC, Wentz A, Abraham AG, Mendley SR, Hooper SR, Butler RW, Gipson DS, Lande MB, Shinnar S, Moxey-Mims MM, Warady BA, Furth SL (2010) Health-related quality of life of children with mild to moderate chronic kidney disease. Pediatrics 125:e349–e357
Goldstein SL (2006) Pediatric acute kidney injury: it’s time for real progress. Pediatr Nephrol 21:891–895
Matza LS, Swensen AR, Flood EM, Secnik K, Leidy NK (2004) Assessment of health-related quality of life in children: a review of conceptual, methodological, and regulatory issues. Value Health 7:79–92
Goldstein SL, Graham N, Warady BA, Seikaly M, McDonald R, Burwinkle TM, Limbers CA, Varni JW (2008) Measuring health-related quality of life in children with ESRD: performance of the generic and ESRD-specific instrument of the Pediatric quality of life inventory (PedsQL). Am J Kidney Dis 51:285–297
Acknowledgments
The authors wish to thank Holly Tomlin (employee and stockholder, Amgen Inc.) for medical writing assistance. The initial drafts of the Introduction and Discussion were written by the first and senior authors, and all authors contributed to the development of the manuscript, provided substantial edits to the drafts and approved the final version of the manuscript in accordance with ICMJE criteria for authorship. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (revised 2005). A signed informed consent form by the patient’s parent or guardian was required for all patients before randomization by an interactive voice response system. A signed assent form was requested from patients ≥7 years old.
Conflict of interest
This study was funded by Amgen Inc. Authors K.O. and J.P. are employees and stockholders of Amgen Inc. B.A.W., J.B., N.B., A.J., L.P., A.S., P.S. and C.J.W. were investigators when the study was conducted.
Rights and permissions
About this article
Cite this article
Warady, B.A., Barcia, J., Benador, N. et al. De novo weekly and biweekly darbepoetin alfa dosing in pediatric patients with chronic kidney disease. Pediatr Nephrol 33, 125–137 (2018). https://doi.org/10.1007/s00467-017-3758-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3758-5