Abstract
Background
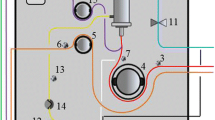
We automated our manual, syringe-driven extracorporeal renal replacement therapy (eRRT) system with an ultra-small volume circuit (3.2 ml) that is suitable for neonates without blood priming. Our objective was to determine the solute clearance and water balance of the automated and manual systems in vitro.
Methods
Stored whole blood samples containing exogenous urea, creatinine (Cr), potassium (K), and ammonia (NH3) to imitate acute kidney injury (AKI) and hyperammonemia were dialyzed for 3 h (blood flow, 4.0 ml/min; dialysate flow, 600 ml/h) with a continuous infusion of heparin. Solute clearance and sample weight were then compared with values before dialysis.
Results
The median clearance of blood urea nitrogen, Cr, K, and NH3 ranged from 1.7 to 2.3 and from 2.4 to 2.6 ml/min, and the median weight of the samples was decreased by 3.8 g and increased by 8.3 g after 3 h of dialysis using the manual and automated systems, respectively.
Conclusions
The automated system effectively cleared solutes, but safety concerns were associated with platelet consumption and fluid balance. Additional studies are needed to establish the safety and accuracy of this novel system for clinical use in neonates and preterm infants.





Similar content being viewed by others
References
Gouyon JB, Guignard JP (2000) Management of acute renal failure in newborns. Pediatr Nephrol 14:1037–1044
Fleming GM, Walters S, Goldstein SL, Alexander SR, Baum MA, Blowey DL, Bunchman TE, Chua AN, Fletcher SA, Flores FX, Fortenberry JD, Hackbarth R, McBryde K, Somers MJG, Symons JM, Brophy PD (2012) Nonrenal indications for continuous renal replacement therapy: a report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry Group. Pediatr Crit Care Med 13:e299–e304
Goldstein SL (2011) Advances in pediatric renal replacement therapy for acute kidney injury. Semin Dial 24:187–191
Santiago MJ, López-Herce J, Urbano J, Solana MJ, del Castillo J, Ballestero Y, Botrán M, Bellón JM (2009) Complications of continuous renal replacement therapy in critically ill children: a prospective observational evaluation study. Crit Care 13:R184
Hackbarth RM, Eding D, Gianoli Smith C, Koch A, Sanfilippo DJ, Bunchman TE (2005) Zero balance ultrafiltration (Z-BUF) in blood-primed CRRT circuits achieves electrolyte and acid–base homeostasis prior to patient connection. Pediatr Nephrol 20:1328–1333
Secher EL, Stensballe J, Afshari A (2013) Transfusion in critically ill children: an ongoing dilemma. Acta Anaesthesiol Scand 57:684–691
Kopp KF, Gutch CF, Kolff WJ (1972) Single needle dialysis. Trans Am Soc Artif Intern Organs 18:75–81
Nishimi S, Ishikawa K, Oda S, Furukawa H, Takada A, Chida S (2015) In vitro ability of a novel system for neonatal extracorporeal renal replacement therapy with ultra-small volume circuit for removing solutes. J Iwate Med Assoc 67:215–223
Bunchman TE, Donckerwolcke RA (1994) Continuous arterial-venous diahemofiltration and continuous veno-venous diahemofiltration in infants and children. Pediatr Nephrol 8:96–102
Ronco C, Garzotto F, Ricci Z (2012) CA.R.PE.DI.E.M (Cardio-Renal Pediatric Dialysis Emergency Machine): evolution of continuous renal replacement therapies in infants. A personal journey. Pediatr Nephrol 27:1203–1211
Coulthard MG, Crosier J, Griffiths C, Smith J, Drinnan M, Whitaker M, Beckwith R, Matthews JNS, Flecknell P, Lambert HJ (2014) Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 29:1873–1881
Everdell NL, Coulthard MG, Crosier J, Keir MJ (2005) A machine for haemodialysing very small infants. Pediatr Nephrol 20:636–643
Everdell NL (2007) A haemodialysis system for the treatment of acute renal failure and metabolic disorders in neonates. Med Eng Phys 29:516–524
Coulthard MG, Sharp J (1995) Haemodialysis and ultrafiltration in babies weighing under 1000 g. Arch Dis Child Fetal Neonatal Ed 73:F162–F165
De Virgiliis G, Vanin M, Buoncristiani U (1985) A new single-needle dialysis system. Trans Am Soc Artif Intern Organs 31:116–118
Murray PT, Brady HR, Hall JB (2006) Intensive care in nephrology. Taylor & Francis, UK, p 204
Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, Chiarenza F, Ricci Z, Goldstein SL (2014) Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CA.R.PE.DI.E.M). Lancet 383:1807–1813
Daugirdas JT, Bernardo AA (2012) Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int 82:147–157
Kiaii M, Djurdjev O, Farah M, Levin A, Jung B, MacRae J (2011) Use of electron-beam sterilized hemodialysis membranes and risk of thrombocytopenia. JAMA 306:1679–1687
Mulder J, Tan HK, Bellomo R, Silvester W (2003) Platelet loss across the hemofilter during continuous hemofiltration. Int J Artif Organs 26:906–912
Acknowledgments
We are grateful to the Mother and Child Health Foundation (Grant No. 23–9). We also thank Asahi Kasei Medical Co., Ltd. for supplying the small hemofilters.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Ethics statement
Written informed consent was obtained from all volunteers to participate in this study, which was approved by the Independent Ethics Committee at Iwate Medical University (approval number: H22-33).
Rights and permissions
About this article
Cite this article
Nishimi, S., Ishikawa, K., Sasaki, M. et al. Ability of a novel system for neonatal extracorporeal renal replacement therapy with an ultra-small volume circuit to remove solutes in vitro . Pediatr Nephrol 31, 493–500 (2016). https://doi.org/10.1007/s00467-015-3233-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3233-0