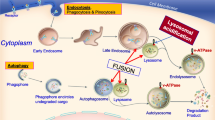
Abstract
The lysosome, an organelle central to macromolecule degradation and recycling, plays a pivotal role in normal cell processes, ranging from autophagy to redox regulation. Not surprisingly, lysosomes are an integral part of the renal epithelial molecular machinery that facilitates normal renal physiology. Two inherited diseases that manifest as kidney dysfunction are Fabry’s disease and cystinosis, each of which is caused by a primary biochemical defect at the lysosome resulting from loss-of-function mutations in genes that encode lysosomal proteins. The functions of the lysosomes in the kidney and how lysosomal dysfunction might contribute to Fabry’s disease and cystinosis are discussed. Unlike most other pediatric renal diseases, therapies are available for Fabry’s disease and cystinosis, but require early diagnosis. Recent analysis of ceroid neuronal lipofuscinosis type 3 (Cln3) null mice, a mouse model of lysosomal disease that is primarily associated with neurological deficits, revealed renal functional abnormalities. As current and future therapeutics increase the life-span of those suffering from diseases like neuronal ceroid lipofuscinosis, it remains a distinct possibility that many more lysosomal disorders that primarily manifest as infant and juvenile neurodegenerative diseases may also include renal disease phenotypes.
Similar content being viewed by others
References
Fogo AB, Bostad L, Svarstad E, Cook WJ, Moll S, Barbey F, Geldenhuys L, West M, Ferluga D, Vujkovac B, Howie AJ, Burns A, Reeve R, Waldek S, Noel LH, Grunfeld JP, Valbuena C, Oliveira JP, Muller J, Breunig F, Zhang X, Warnock DG, all members of the International Study Group of Fabry N (2010) Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant 25:2168–2177
Alroy J, Sabnis S, Kopp JB (2002) Renal pathology in Fabry disease. J Am Soc Nephrol 13:S134–S138
Valbuena C, Carvalho E, Bustorff M, Ganhao M, Relvas S, Nogueira R, Carneiro F, Oliveira JP (2008) Kidney biopsy findings in heterozygous Fabry disease females with early nephropathy. Virchows Arch 453:329–338
Gubler MC, Lenoir G, Grunfeld JP, Ulmann A, Droz D, Habib R (1978) Early renal changes in hemizygous and heterozygous patients with Fabry's disease. Kidney Int 13:223–235
Gahl WA, Thoene JG, Schneider JA (2002) Cystinosis. N Engl J Med 347:111–121
Weidemann F, Sanchez-Nino MD, Politei J, Oliveira JP, Wanner C, Warnock DG, Ortiz A (2013) Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis 8:116
Saito A, Sato H, Iino N, Takeda T (2010) Molecular mechanisms of receptor-mediated endocytosis in the renal proximal tubular epithelium. J Biomed Biotechnol 2010:403272
Weyer K, Storm T, Shan J, Vainio S, Kozyraki R, Verroust PJ, Christensen EI, Nielsen R (2011) Mouse model of proximal tubule endocytic dysfunction. Nephrol Dial Transplant 26:3446–3451
Christensen EI, Birn H (2002) Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3:256–266
Balreira A, Gaspar P, Caiola D, Chaves J, Beirao I, Lima JL, Azevedo JE, Miranda MC (2008) A nonsense mutation in the LIMP-2 gene associated with progressive myoclonic epilepsy and nephrotic syndrome. Hum Mol Genet 17:2238–2243
Shrimpton AE, Hoopes RR Jr, Knohl SJ, Hueber P, Reed AA, Christie PT, Igarashi T, Lee P, Lehman A, White C, Milford DV, Sanchez MR, Unwin R, Wrong OM, Thakker RV, Scheinman SJ (2009) OCRL1 mutations in Dent 2 patients suggest a mechanism for phenotypic variability. Nephron Physiol 112:p27–p36
Yamamoto K, Cox JP, Friedrich T, Christie PT, Bald M, Houtman PN, Lapsley MJ, Patzer L, Tsimaratos M, Van THWG, Yamaoka K, Jentsch TJ, Thakker RV (2000) Characterization of renal chloride channel (CLCN5) mutations in Dent's disease. J Am Soc Nephrol 11:1460–1468
Christensen EI, Devuyst O, Dom G, Nielsen R, Van der Smissen P, Verroust P, Leruth M, Guggino WB, Courtoy PJ (2003) Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci U S A 100:8472–8477
Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ (2000) ClC-5 Cl- -channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature 408:369–373
Nielsen R, Courtoy PJ, Jacobsen C, Dom G, Lima WR, Jadot M, Willnow TE, Devuyst O, Christensen EI (2007) Endocytosis provides a major alternative pathway for lysosomal biogenesis in kidney proximal tubular cells. Proc Natl Acad Sci U S A 104:5407–5412
Vicinanza M, Di Campli A, Polishchuk E, Santoro M, Di Tullio G, Godi A, Levtchenko E, De Leo MG, Polishchuk R, Sandoval L, Marzolo MP, De Matteis MA (2011) OCRL controls trafficking through early endosomes via PtdIns4,5P2-dependent regulation of endosomal actin. EMBO J 30:4970–4985
Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M (2005) Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell 16:3467–3479
Desmond MJ, Lee D, Fraser SA, Katerelos M, Gleich K, Martinello P, Li YQ, Thomas MC, Michelucci R, Cole AJ, Saftig P, Schwake M, Stapleton D, Berkovic SF, Power DA (2011) Tubular proteinuria in mice and humans lacking the intrinsic lysosomal protein SCARB2/Limp-2. Am J Physiol Ren Physiol 300:F1437–F1447
Schroder J, Lullmann-Rauch R, Himmerkus N, Pleines I, Nieswandt B, Orinska Z, Koch-Nolte F, Schroder B, Bleich M, Saftig P (2009) Deficiency of the tetraspanin CD63 associated with kidney pathology but normal lysosomal function. Mol Cell Biol 29:1083–1094
Wesche D, Deen PM, Knoers NV (2012) Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol 27:2183–2204
Lerner TJ, Boustany R-MN, Anderson JW, D’Arigo KL, Schlumpf K, Buckler AJ, Gusella JF, Haines JL (1995) Isolation of a novel gene underlying Batten disease, CLN3. The International Batten Disease Consortium. Cell 82:949–957
Pontikis CC, Cella CV, Parihar N, Lim MJ, Chakrabarti S, Mitchison HM, Mobley WC, Rezaie P, Pearce DA, Cooper JD (2004) Late onset neurodegeneration in the Cln3−/− mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res 1023:231–242
Stein CS, Yancey PH, Martins I, Sigmund RD, Stokes JB, Davidson BL (2010) Osmoregulation of ceroid neuronal lipofuscinosis type 3 in the renal medulla. Am J Physiol Cell Physiol 298:C1388–C1400
Sasaki S, Chiga M, Kikuchi E, Rai T, Uchida S (2013) Hereditary nephrogenic diabetes insipidus in Japanese patients: analysis of 78 families and report of 22 new mutations in AVPR2 and AQP2. Clin Exp Nephrol 17:338–344
Zhou B, Zhuang J, Gu D, Wang H, Cebotaru L, Guggino WB, Cai H (2010) WNK4 enhances the degradation of NCC through a sortilin-mediated lysosomal pathway. J Am Soc Nephrol 21:82–92
McCormick JA, Ellison DH (2011) The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 91:177–219
Christensen EI, Zhou Q, Sorensen SS, Rasmussen AK, Jacobsen C, Feldt-Rasmussen U, Nielsen R (2007) Distribution of alpha-galactosidase A in normal human kidney and renal accumulation and distribution of recombinant alpha-galactosidase A in Fabry mice. J Am Soc Nephrol 18:698–706
Valbuena C, Oliveira JP, Carneiro F, Relvas S, Ganhao M, Sa-Miranda MC, Rodrigues LG (2011) Kidney histologic alterations in alpha-Galactosidase-deficient mice. Virchows Arch 458:477–486
Liu Y (2011) Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7:684–696
Fischer EG, Moore MJ, Lager DJ (2006) Fabry disease: a morphologic study of 11 cases. Mod Pathol 19:1295–1301
Mather AR, Siskind LJ (2011) Glycosphingolipids and kidney disease. Adv Exp Med Biol 721:121–138
Chao CT, Lin WC, Kao TW (2012) Fabry disease and immunoglobulin A nephropathy. Nephrology 17:782–783
Martinez P, Aggio M, Rozenfeld P (2007) High incidence of autoantibodies in Fabry disease patients. J Inherit Metab Dis 30:365–369
Maixnerova D, Tesar V, Rysava R, Reiterova J, Poupetova H, Dvorakova L, Golan L, Neprasova M, Kidorova J, Merta M, Honsova E (2013) The coincidence of IgA nephropathy and Fabry disease. BMC Nephrol 14:6
Shimohata H, Yoh K, Takada K, Tanaka H, Usui J, Hirayama K, Kobayashi M, Yamagata K (2009) Hemizygous Fabry disease associated with IgA nephropathy: a case report. J Nephrol 22:682–684
Whybra C, Schwarting A, Kriegsmann J, Gal A, Mengel E, Kampmann C, Baehner F, Schaefer E, Beck M (2006) IgA nephropathy in two adolescent sisters heterozygous for Fabry disease. Pediatr Nephrol 21:1251–1256
Schneider JA, Bradley K, Seegmiller JE (1967) Increased cystine in leukocytes from individuals homozygous and heterozygous for cystinosis. Science 157:1321–1322
Schneider JA, Rosenbloom FM, Bradley KH, Seegmiller JE (1967) Increased free-cystine content of fibroblasts cultured from patients with cystinosis. Biochem Biophys Res Commun 29:527–531
Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van't Hoff W, Antignac C (1998) A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet 18:319–324
Schulman JD, Bradley KH, Seegmiller JE (1969) Cystine: compartmentalization within lysosomes in cystinotic leukocytes. Science 166:1152–1154
Jonas AJ, Smith ML, Schneider JA (1982) ATP-dependent lysosomal cystine efflux is defective in cystinosis. J Biol Chem 257:13185–13188
Kalatzis V, Cherqui S, Antignac C, Gasnier B (2001) Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J 20:5940–5949
Attard M, Jean G, Forestier L, Cherqui S, van't Hoff W, Broyer M, Antignac C, Town M (1999) Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum Mol Genet 8:2507–2514
Nesterova G, Gahl WA (2013) Cystinosis: the evolution of a treatable disease. Pediatr Nephrol 28:51–59
Darmady EM, Stranack F (1957) Microdissection of the nephron in disease. Br Med Bull 13:21–26
Mahoney CP, Striker GE (2000) Early development of the renal lesions in infantile cystinosis. Pediatr Nephrol 15:50–56
Schneider JA, Clark KF, Greene AA, Reisch JS, Markello TC, Gahl WA, Thoene JG, Noonan PK, Berry KA (1995) Recent advances in the treatment of cystinosis. J Inherit Metab Dis 18:387–397
Sansanwal P, Sarwal MM (2012) p62/SQSTM1 prominently accumulates in renal proximal tubules in nephropathic cystinosis. Pediatr Nephrol 27:2137–2144
Sansanwal P, Yen B, Gahl WA, Ma Y, Ying L, Wong LJ, Sarwal MM (2010) Mitochondrial autophagy promotes cellular injury in nephropathic cystinosis. J Am Soc Nephrol 21:272–283
Christensen EI, Gburek J (2004) Protein reabsorption in renal proximal tubule-function and dysfunction in kidney pathophysiology. Pediatr Nephrol 19:714–721
Johnson JL, Napolitano G, Monfregola J, Rocca CJ, Cherqui S, Catz SD (2013) Upregulation of the rab27a-dependent trafficking and secretory mechanisms improves lysosomal transport, alleviates endoplasmic reticulum stress, and reduces lysosome overload in cystinosis. Mol Cell Biol 33:2950–2962
Maejima I, Takahashi A, Omori H, Kimura T, Takabatake Y, Saitoh T, Yamamoto A, Hamasaki M, Noda T, Isaka Y, Yoshimori T (2013) Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J 32:2336–2347
Wilmer MJ, Emma F, Levtchenko EN (2010) The pathogenesis of cystinosis: mechanisms beyond cystine accumulation. Am J Physiol Ren Physiol 299:F905–F916
Greene AA, Jonas AJ, Harms E, Smith ML, Pellett OL, Bump EA, Miller AL, Schneider JA (1985) Lysosomal cystine storage in cystinosis and mucolipidosis type II. Pediatr Res 19:1170–1174
Park M, Helip-Wooley A, Thoene J (2002) Lysosomal cystine storage augments apoptosis in cultured human fibroblasts and renal tubular epithelial cells. J Am Soc Nephrol 13:2878–2887
Wilmer MJ, de Graaf-Hess A, Blom HJ, Dijkman HB, Monnens LA, van den Heuvel LP, Levtchenko EN (2005) Elevated oxidized glutathione in cystinotic proximal tubular epithelial cells. Biochem Biophys Res Commun 337:610–614
Mannucci L, Pastore A, Rizzo C, Piemonte F, Rizzoni G, Emma F (2006) Impaired activity of the gamma-glutamyl cycle in nephropathic cystinosis fibroblasts. Pediatr Res 59:332–335
Chol M, Nevo N, Cherqui S, Antignac C, Rustin P (2004) Glutathione precursors replenish decreased glutathione pool in cystinotic cell lines. Biochem Biophys Res Commun 324:231–235
Coor C, Salmon RF, Quigley R, Marver D, Baum M (1991) Role of adenosine triphosphate (ATP) and NaK ATPase in the inhibition of proximal tubule transport with intracellular cystine loading. J Clin Invest 87:955–961
Levtchenko EN, Wilmer MJ, Janssen AJ, Koenderink JB, Visch HJ, Willems PH, de Graaf-Hess A, Blom HJ, van den Heuvel LP, Monnens LA (2006) Decreased intracellular ATP content and intact mitochondrial energy generating capacity in human cystinotic fibroblasts. Pediatr Res 59:287–292
Kumar A, Bachhawat AK (2010) A futile cycle, formed between two ATP-dependant gamma-glutamyl cycle enzymes, gamma-glutamyl cysteine synthetase and 5-oxoprolinase: the cause of cellular ATP depletion in nephrotic cystinosis? J Biosci 35:21–25
Laube GF, Shah V, Stewart VC, Hargreaves IP, Haq MR, Heales SJ, van't Hoff WG (2006) Glutathione depletion and increased apoptosis rate in human cystinotic proximal tubular cells. Pediatr Nephrol 21:503–509
Wilmer MJ, Kluijtmans LA, van der Velden TJ, Willems PH, Scheffer PG, Masereeuw R, Monnens LA, van den Heuvel LP, Levtchenko EN (2011) Cysteamine restores glutathione redox status in cultured cystinotic proximal tubular epithelial cells. Biochim Biophys Acta 1812:643–651
Jackson JD, Smith FG, Litman NN, Yuile CL, Latta H (1962) The Fanconi syndrome with cystinosis. Electron microscopy of renal biopsy specimens from five patients. Am J Med 33:893–910
Park MA, Pejovic V, Kerisit KG, Junius S, Thoene JG (2006) Increased apoptosis in cystinotic fibroblasts and renal proximal tubule epithelial cells results from cysteinylation of protein kinase Cdelta. J Am Soc Nephrol 17:3167–3175
Gao XD, Wang J, Keppler-Ross S, Dean N (2005) ERS1 encodes a functional homologue of the human lysosomal cystine transporter. FEBS J 272:2497–2511
Bechtel W, Helmstadter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, Schell C, Kretz O, Liu S, Geist F, Kerjaschki D, Walz G, Huber TB (2013) Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J Am Soc Nephrol 24:727–743
Chen J, Chen MX, Fogo AB, Harris RC, Chen JK (2013) mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J Am Soc Nephrol 24:198–207
Najafian B, Mauer M, Hopkin RJ, Svarstad E (2013) Renal complications of Fabry disease in children. Pediatr Nephrol 28:679–687
Pisoni RL, Thoene JG, Christensen HN (1985) Detection and characterization of carrier-mediated cationic amino acid transport in lysosomes of normal and cystinotic human fibroblasts. Role in therapeutic cystine removal? J Biol Chem 260:4791–4798
Markello TC, Bernardini IM, Gahl WA (1993) Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med 328:1157–1162
Nesterova G, Gahl W (2008) Nephropathic cystinosis: late complications of a multisystemic disease. Pediatr Nephrol 23:863–878
Levtchenko EN, van Dael CM, de Graaf-Hess AC, Wilmer MJ, van den Heuvel LP, Monnens LA, Blom HJ (2006) Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr Nephrol 21:110–113
Dohil R, Cabrera BL (2013) Treatment of cystinosis with delayed-release cysteamine: 6-year follow-up. Pediatr Nephrol 28:507–510
Harrison F, Yeagy BA, Rocca CJ, Kohn DB, Salomon DR, Cherqui S (2013) Hematopoietic stem cell gene therapy for the multisystemic lysosomal storage disorder cystinosis. Mol Ther 21:433–444
Cherqui S, Kalatzis V, Trugnan G, Antignac C (2001) The targeting of cystinosin to the lysosomal membrane requires a tyrosine-based signal and a novel sorting motif. J Biol Chem 276:13314–13321
Nevo N, Chol M, Bailleux A, Kalatzis V, Morisset L, Devuyst O, Gubler MC, Antignac C (2010) Renal phenotype of the cystinosis mouse model is dependent upon genetic background. Nephrol Dial Transplant 25:1059–1066
Getty A, Kovacs AD, Lengyel-Nelson T, Cardillo A, Hof C, Chan CH, Pearce DA (2013) Osmotic stress changes the expression and subcellular localization of the Batten disease protein CLN3. PloS ONE 8:e66203
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001) Human hypertension caused by mutations in WNK kinases. Science 293:1107–1112
Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S (2007) Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5:331–344
Gamp AC, Tanaka Y, Lullmann-Rauch R, Wittke D, D'Hooge R, De Deyn PP, Moser T, Maier H, Hartmann D, Reiss K, Illert AL, von Figura K, Saftig P (2003) LIMP-2/LGP85 deficiency causes ureteric pelvic junction obstruction, deafness and peripheral neuropathy in mice. Hum Mol Genet 12:631–646
Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, Thakker RV, Guggino S, Guggino WB (2000) Mice lacking renal chloride channel, CLC-5, are a model for Dent's disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet 9:2937–2945
Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, Weinreb RN, Travers JB, Khanna H, Sun Y (2012) OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome. Hum Mol Genet 21:3333–3344
Bothwell SP, Chan E, Bernardini IM, Kuo YM, Gahl WA, Nussbaum RL (2011) Mouse model for Lowe syndrome/Dent Disease 2 renal tubulopathy. J Am Soc Nephrol 22:443–448
Pols MS, Klumperman J (2009) Trafficking and function of the tetraspanin CD63. Exp Cell Res 315:1584–1592
Acknowledgements
Partially supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 1P20GM103620-01A1 (Pediatrics) and by Sanford Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Surendran, K., Vitiello, S.P. & Pearce, D.A. Lysosome dysfunction in the pathogenesis of kidney diseases. Pediatr Nephrol 29, 2253–2261 (2014). https://doi.org/10.1007/s00467-013-2652-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2652-z