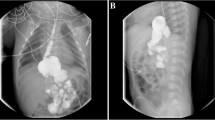
Abstract
The Wilms’ tumor suppressor gene WT1 is an important regulator of development. Mutations in this gene have been associated with Wilms’ tumor, Frasier syndrome, and Denys–Drash syndrome, as well as isolated glomerular disease. Here we report the case of a 4-month-old girl, who presented with end-stage renal disease, thrombopenia, anemia, and cardiac hypertrophy accompanied by severe hypertension. Histological analysis of kidney biopsies revealed a massive and diffuse nephroblastomatosis with a dramatic reduction in the number of glomeruli. Although no normal cortical nephrons could be detected, medullary organization was nearly normal. Sequence analysis demonstrated a heterozygous nonsense mutation in exon 9 of WT1, which leads to a truncation of the WT1 protein at the beginning of zinc finger 3. Given the requirement of WT1 for normal development of the kidney and heart, these data raise the hypothesis that the mutation identified was responsible for the severe phenotype observed in our patient.





Similar content being viewed by others
References
Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R (1993) WT-1 is required for early kidney development. Cell 74:679–691
Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A (1999) YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 126:1845–1857
Wagner N, Wagner KD, Theres H, Englert C, Schedl A, Scholz H (2005) Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms’ tumor transcription factor Wt1. Genes Dev 19:2631–2642
Herzer U, Crocoll A, Barton D, Howells N, Englert C (1999) The Wilms tumor suppressor gene wt1 is required for development of the spleen. Curr Biol 9:837–840
Wagner KD, Wagner N, Vidal VP, Schley G, Wilhelm D, Schedl A, Englert C, Scholz H (2002) The Wilms’ tumor gene Wt1 is required for normal development of the retina. EMBO J 21:1398–1405
Wagner N, Wagner KD, Hammes A, Kirschner KM, Vidal VP, Schedl A, Scholz H (2005) A splice variant of the Wilms’ tumor suppressor Wt1 is required for normal development of the olfactory system. Development 132:1327–1336
Davies JA, Ladomery M, Hohenstein P, Michael L, Shafe A, Spraggon L, Hastie N (2004) Development of an siRNA-based method for repressing specific genes in renal organ culture and its use to show that the Wt1 tumor suppressor is required for nephron differentiation. Hum Mol Genet 13:235–246
Guo JK, Menke AL, Gubler MC, Clarke AR, Harrison D, Hammes A, Hastie ND, Schedl A (2002) WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet 11:651–659
Niaudet P, Gubler MC (2006) WT1 and glomerular diseases. Pediatr Nephrol 21:1653–1660
Wagner KD, Wagner N, Bondke A, Nafz B, Flemming B, Theres H, Scholz H (2002) The Wilms’ tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction. FASEB J 16:1117–1119
Haber DA, Buckler AJ, Glaser T, Call KM, Pelletier J, Sohn RL, Douglass EC, Housman DE (1990) An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms’ tumor. Cell 61:1257–1269
Gessler M, Poustka A, Cavenee W, Neve RL, Orkin SH, Bruns GA (1990) Homozygous deletion in Wilms tumors of a zinc-finger gene identified by chromosome jumping. Nature 343:774–778
Pelletier J, Bruening W, Li FP, Haber DA, Glaser T, Housman DE (1991) WT1 mutations contribute to abnormal genital system development and hereditary Wilms’ tumor. Nature 353:431–434
Klamt B, Koziell A, Poulat F, Wieacker P, Scambler P, Berta P, Gessler M (1998) Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1+/−KTS splice isoforms. Hum Mol Genet 7:709–714
Barbaux S, Niaudet P, Gubler MC, Grunfeld JP, Jaubert F, Kuttenn F, Fekete CN, Souleyreau Therville N, Thibaud E, Fellous M, McElreavey K (1997) Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet 17:467–470
Wagner N, Panelos J, Massi D, Wagner KD (2008) The Wilms’ tumor suppressor WT1 is associated with melanoma proliferation. Pflugers Arch 455:839–847
White KS, Kirks DR, Bove KE (1992) Imaging of nephroblastomatosis. An overview. Radiology 82:1–5
Finegold M, Bennington JL (1986) Pathology of neoplasms of children and adolescents, Saunders, Philadelphia
Wagner N, Wagner KD, Scholz H, Kirschner KM, Schedl A (2006) The intermediate filament protein nestin is expressed in the developing kidney and heart and might be regulated by the Wilms’ tumor suppressor Wt1. Am J Physiol Regul Integr Comp Physiol 291:R779–R787
Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K (1998) Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1:575–582
Wagner N, Wagner KD, Xing Y, Scholz H, Schedl A (2004) The major podocyte protein nephrin is transcriptionally activated by the Wilms’ tumor suppressor WT1. J Am Soc Nephrol 15:3044–3051
Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C (2000) NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24:349–354
Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W (1997) Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139:193–204
Royer-Pokora B, Beier M, Henzler M, Alam R, Schumacher V, Weirich A, Huff V (2004) Twenty-four new cases of WT1 germline mutations and review of the literature: genotype/phenotype correlations for Wilms tumor development. Am J Med Genet 127:249–257
Acknowledgments
The authors thank Marie Claire Gubler for the kidney control tissue of a 2-month-old child. This work was supported by grants from Fondation pour la Recherche médicale (FRM), EuReGene (EU), FP6 and L’Agence Nationale de la Recherche (ANR) (ANR-05-MRAR-019-01). N.W. is a fellow of Fondation - de France.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wagner, N., Wagner, KD., Afanetti, M. et al. A novel Wilms’ tumor 1 gene mutation in a child with severe renal dysfunction and persistent renal blastema. Pediatr Nephrol 23, 1445–1453 (2008). https://doi.org/10.1007/s00467-008-0845-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0845-7