Abstract
Background
Despite there being a considerable amount of published studies on robotic colorectal surgery (RCS) over the last few years, there is a lack of evidence regarding RCS training pathways. This study examines the short-term clinical outcomes of an international RCS training programme (the European Academy of Robotic Colorectal Surgery—EARCS).
Methods
Consecutive cases from 26 European colorectal units who conducted RCS between 2014 and 2018 were included in this study. The baseline characteristics and short-term outcomes of cases performed by EARCS delegates during training were analysed and compared with cases performed by EARCS graduates and proctors.
Results
Data from 1130 RCS procedures were collected and classified into three cohort groups (323 training, 626 graduates and 181 proctors). The training cases conversion rate was 2.2% and R1 resection rate was 1.5%. The three groups were similar in terms of baseline characteristics with the exception of malignant cases and rectal resections performed. With the exception of operative time, blood loss and hospital stay (training vs. graduate vs. proctor: operative time 302, 265, 255 min, p < 0.001; blood loss 50, 50, 30 ml, p < 0.001; hospital stay 7, 6, 6 days, p = 0.003), all remaining short-term outcomes (conversion, 30-day reoperation, 30-day readmission, 30-day mortality, clinical anastomotic leak, complications, R1 resection and lymph node yield) were comparable between the three groups.
Conclusions
Colorectal surgeons learning how to perform RCS under the EARCS-structured training pathway can safely achieve short-term clinical outcomes comparable to their trainers and overcome the learning process in a way that minimises patient harm.
Similar content being viewed by others
Since the first robotic colectomy in 2002 [1], robotic surgery has increasingly gained acceptance in colorectal surgery, which is evident from the growing number of studies published on the subject [2,3,4] and the increasing number of robotic units acquired worldwide [5]. Robotic platforms are reported to offer advantages such as stable 3D views, tremor filtering and angulated instruments with multiple degrees of freedom which may explain this increasing popularity [6, 7].
Although there is convincing evidence to support the safety and feasibility of robotic colorectal surgery, with short and long-term outcomes similar or possibly even superior to those of laparoscopic surgery [8,9,10,11,12,13,14,15,16,17], there is little evidence to show how this new technology may be safely implemented for colorectal surgery. For new technologies and methods of operating to be introduced, appropriate training remains imperative in order to ensure patient safety and improved clinical outcomes [18]. Most specialist surgeons depend on attending short courses or visiting centres of excellence in order to gain competence in a new surgical technique [19]. However, without a structured training pathway, surgeons may run the risk of exposing their patients to an increasing number of complications during the learning process. In the UK, LAPCO [20], a national training programme designed for the safe adoption of laparoscopic colorectal surgery, led to improved clinical outcomes and minimised patient harm, while surgeons were gaining competency in laparoscopic colorectal surgery [19]. Recognising such a need for robotic colorectal surgery, the European Academy of Robotic Colorectal Surgery (EARCS) was founded (https://earcs.pt) under the auspices of the Champalimaud Foundation in Lisbon, Portugal. EARCS provides a framework and guidelines for selecting appropriate surgeons, skill courses and direct supervision of clinical cases for robotic colorectal surgery. EARCS is audited by collecting clinical results, while surgical performance is evaluated using a structured process for formative and summative assessment. Since October 2014, a total of 148 surgeons have registered with the training programme and 76 surgeons from 54 centres in 15 European countries have graduated from the academy [21].
The aim of this study is to examine the short-term outcomes of a structured training programme for robotic colorectal surgery in an international setting. The presented data give an insight into the training experience of robotic colorectal surgeons across Europe and addresses the paucity of evidence in robotic colorectal surgery training methods on the path to attaining independent practice.
Materials and methods
Consecutive cases from 26 European colorectal units who performed robotic colorectal surgery between 2014 and 2018 were included in this study. Each centre prospectively collected the baseline characteristics and short-term outcomes of all participating patients. The inclusion criteria included all elective patients eligible for robotic colorectal surgery. Non-colorectal cases were excluded.
Cases were either performed by an EARCS proctor, an EARCS graduate or as part of a training case where an EARCS proctor would train an EARCS delegate. A total of 35 surgeons participated in this study with 7 proctors supervising all training cases. The short-term clinical and oncological outcomes of all cases performed as part of a training case were analysed and compared with cases performed by an EARCS graduate or proctor as a control. Additionally, conversion and complication cumulative sum (CUSUM) charts for the first 10 training cases were constructed to identify whether there was a change in outcome trend during training.
Cancer cases followed local pre and post-operative guidelines and were discussed in the institutional multidisciplinary team meeting (MDT) prior to initiating any type of treatment. Neoadjuvant treatment was given according to local guidelines following MDT discussion. Modified enhanced recovery programmes after surgery (ERAS) were used as standard at all colorectal units in this study [22]. The decision on whether to perform the surgeries robotically was based on surgeon discretion as there were no set criteria for robotic surgery allocation. The majority of the participating centres used the robot for rectal cancer cases only. Robotic surgeries were either performed with the da Vinci Xi, da Vinci Si or da Vinci X models. A standardised fully robotic single docking technique was applied for all surgeries as taught in the EARCS programme [23, 24]. With the exception of robotic setup and docking, the operative modules were the same for all robotic systems.
All included patients signed an informed consent form allowing their data to be used for analysis and research. The requirements for anonymisation of personal dataset by the Data Protection Act 1998 were satisfied.
Training programme
As the programme was designed for established specialist surgeons, it had different characteristics in its structure compared with training schemes for surgical trainees. Expert trainers were identified and appointed to provide training with a national coordinator and a centre for educational assessment and research. As a prerequisite, expert trainers were required to have conducted a minimum of 200 robotic colorectal resections and to submit a double blinded video assessment of their operative technique.
Of note is that laparoscopic surgery experience was not considered as a prerequisite for delegates to be accepted to the programme. Delegates were encouraged to perform at least 30 h of simulation exercises before the training programme commenced, to familiarise themselves with the robotic console. In addition, each surgeon was required to complete the online modules for the robotic system they were going to be trained on.
The final training step involved hands-on training at one of the faculty member’s hospitals, followed by supervised training at the delegate’s own hospital. All cases were performed under the direct supervision of the faculty who provided mentor support. Delegates and proctors both completed a Global Assessment Score (GAS) form after each proctored (training) case [18]. The number of proctored cases required for each delegate varied with the proficiency of the delegate and was decided by the proctors based on the GAS form as a formative assessment. After completing the required number of proctored cases, delegates were expected to take a final assessment by submitting two anonymized and unedited videos of self-performed robotic anterior resections, including splenic flexure mobilisation, for blinded assessment by the EARCS surgical competency assessment committee using the Robotic Colorectal Assessment Tool (RCAT) as a summative assessment.
EARCS did not have a separate programme for colonic and rectal/pelvic dissection. This is because the aim of the programme was to make surgeons competent in both colonic and rectal surgery. As part of the training for rectal surgery, surgeons were assessed on the modules for colonic dissection, including transection of the vascular pedicle, mobilisation of the colon and splenic flexure mobilisation. In terms of the first training cases performed, it was encouraged to start with easier cases, such as sigmoid or rectosigmoid cancers, and then moving onto the lower rectal cancers. However, this was largely dependent on case availability.
Data collection and outcome assessment
All data were collected from prospectively maintained databases from each institution and sent to a central data manager who collated the data. The baseline characteristics and short-term surgical outcomes of all elective patients receiving robotic colorectal surgery were collected and analysed. Baseline characteristics included age, body mass index (BMI), gender, American Society of Anaesthesiologists (ASA) grade, malignant vs. benign surgery, neoadjuvant treatment, pathological T and N stage, and the type of operation performed. Perioperative data included operative time, estimated blood loss (EBL) and conversion to open surgery (defined as any incision needed to either mobilise the colon or rectum or ligate the vessels). Post-operative clinical data examined included length of stay (LOS), post-operative complications—Clavien–Dindo (CD) classification [25], 30-day reoperation (defined as any operation requiring a general anaesthetic within 30 days from surgery), 30-day readmission, 30-day mortality and clinical anastomotic leak (defined as any anastomotic leak requiring another intervention, such as external drainage or reoperation). Oncological outcomes examined by a pathologist included lymph node yield and circumferential resection margin (CRM) clearance. CD complication grades 1–2 and 3–5 were grouped together for the purposes of statistical analysis.
A subgroup analysis was performed for patients receiving robotic rectal resections in order to investigate whether conclusions similar to those of the complete cohort can be reached when specifically looking at rectal resections; therefore, eliminating any bias arising from differences in the nature of the procedures performed between the three groups.
Statistical analysis
Data were analysed using IBM SPSS version 24 (SPSS Inc., Chicago, IL, USA). Non-parametric data were expressed as median with interquartile range and parametric data as mean with standard deviation. When investigating all three cohorts, baseline demographic and clinical characteristics were compared using the χ2 test for categorical variables, Kruskal Wallis test for non-parametric continuous variables and one-way ANOVA for parametric continuous variables. P values of <0.05 were considered statistically significant.
Univariate binary logistic regression analysis was performed to assess whether surgeon role (training, graduate, proctor) affected CD 1–2 and CD 3–5 complication rates. Following this, a multivariate model was applied where surgeon role was adjusted for all clinically relevant variables (gender, age, BMI, ASA grade, rectal resection, neoadjuvant treatment, cancer case). For the purpose of binary logistic regression, missing values were replaced with the series mean (11 missing values for ASA, 53 for BMI). The constant was included in the analysis model and data are presented as odds ratio, 95% confidence interval and p value.
CUSUM curves for conversion rate and CD 3–5 complications were charted in order to assess the trend of these outcomes during the training process [26, 27]. For the construction of the CUSUM charts the target was the conversion and CD 3–5 complication rate of all graduate cases (X0). Therefore, the CUSUM score is the cumulative sum of Xi – X0 where Xi represents the average conversion or CD 3–5 complication rate of each succeeding training case for the first ten cases (i.e., the average of all first training cases, followed by the average of all the second training cases, etc.). In the CUSUM chart the x-axis represents the consecutive cases and the y-axis the CUSUM score. The CUSUM curves ascend when the set target is not reached, which reflects an ongoing learning process. When the curve plateaus or descends the target is achieved or superseded, representing the end of the learning process. CUSUM curves are only charted for the first 10 cases, since after 10 the number of surgeons performing further training cases significantly drops.
Results
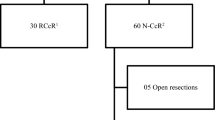
A total of 1130 patients were included in this study. Of those, 323 (28.6%) were EARCS training cases, 626 (55.4%) were performed by EARCS graduates and 181 (16%) by EARCS proctors.
Cohort characteristics
The three groups were similar in terms of baseline characteristics. However, the proctor group had a greater proportion of malignant cases and rectal resections. The baseline characteristics of the three groups are summarised in Table 1.
Short-term outcomes
The short-term outcomes of the three cohorts are detailed in Table 2. There was a relatively low number of conversions, complications, 30-day mortality and R1 resections (for all malignant cases) observed in the training cases, as well as the graduate and proctor cases.
The conversion rate, 30-day reoperation rate, 30-day readmission rate, 30-day mortality rate, clinical anastomotic leak rate, post-operative complications, CRM clearance and lymph node yield did not differ significantly between the three groups.
The median operation time was shorter in the proctor group and longer in the training group (training vs. graduate vs. proctor: 302, 265, 255 min; p < 0.001). The EBL was lower in the proctor group (training vs. graduate vs. proctor: 50, 50, 30 ml; p < 0.001). There was a difference in LOS favouring proctors and graduates, when compared to training cases (training vs. graduate vs. proctor: 7, 6, 6 days; p = 0.003).
Logistic regression analysis for post-operative complications
Univariate logistic regression analysis showed that surgeon role did not affect post-operative CD 1–2 or CD 3–5 complications. This was also the case in the multivariate regression analysis when other clinically relevant factors were adjusted for (gender, age, BMI, ASA grade, rectal resection, neoadjuvant treatment, cancer case). Both univariate and multivariate analysis demonstrated that rectal resection was a risk factor for CD 1–2 and CD 3–5 complications. The logistic regression analysis results for CD 1–2 and CD 3–5 complications are presented in Tables 3 and 4, respectively.
Cumulative sum (CUSUM) charts
The CUSUM charts for conversion (Fig. 1) and complications CD 3–5 (Fig. 2) did not indicate a positive or negative trend over the first 10 cases, demonstrating that these outcomes are maintained on target during the learning process.
Subgroups analysis for patients receiving robotic rectal resections
There were 826 patients that had robotic rectal resections. Of these, 225 (27.2%) were training cases, 448 (54.2%) were performed by a graduate and 153 (18.5%) by a proctor. The baseline characteristics and short-term outcomes are summarised in Tables 5 and 6. The three groups were similar in terms of baseline characteristics, procedures performed and short-term outcomes, with the exception of operation time, EBL and LOS. Operation time was longer in the training group (training vs. graduate vs. proctor: 340.5, 300, 260 min; p < 0.001), EBL was again lower in the proctor group (training vs. graduate vs. proctor: 50, 50, 32.5 ml; p < 0.001) and LOS favoured the proctor and graduate cohorts (training vs. graduate vs. proctor: 7, 6, 6 days; p = 0.044). Univariate and multivariate logistic regression analysis again showed that surgeon role did not affect CD 1–2 or CD 3–5 complications.
Discussion
Robotic surgery is greatly increasing in popularity among the colorectal surgical community. Structured mentorship training programmes are essential for the safe adoption of any new surgical technique. While several studies on robotic colorectal surgery have been published over the last few years, there is a lack of evidence regarding robotic colorectal surgery training pathways. In other specialties such as urology, training schemes for robotic surgery have been described, although there is still a recognised need for a structured, standardised curriculum and robust credentialing guidelines for proficiency [28,29,30,31,32,33]. To our knowledge, in colorectal surgery three studies discuss a training pathway for robotic surgery [18, 34, 35], but only include a relatively small number of surgeons and cases.
This study examines the outcomes of 1130 robotic colorectal cases across 26 centres in Europe and its results suggest that the EARCS programme facilitates safe and effective training of robotic colorectal surgery while ensuring good clinical outcomes. This is demonstrated by examining the short-term surgical outcomes of robotic colorectal surgeries performed by three cohorts: EARCS delegates during training, EARCS graduates and EARCS proctors.
A similar clinical and oncological short-term outcome profile was observed between the three groups, with the exception of operative time, EBL and LOS. This suggests that colorectal surgeons in training, learning how to perform robotic colorectal surgery under the EARCS-structured training pathway, can safely achieve short-term surgical outcomes comparable to those of the EARCS graduates and proctors. This was also confirmed in binary logistic regression analysis where surgeon role (training vs. graduate vs. proctor) was not found to affect CD 1–2 or CD 3–5 complications (see Tables 3 and 4). Additionally, the short-term outcomes of the EARCS graduate and proctor cohorts did not differ significantly, including operation time and length of stay, with the exception of EBL which was lower in the proctor group. The inference that EARCS graduates can achieve short-term outcomes similar to those who trained them is suggestive of the effectiveness of the training programme. Finally, CUSUM graph analysis of the first 10 training cases for conversion rate and CD 3–5 complications, showed no clear positive or negative trend, demonstrating that patient outcomes are stable during the learning process.
The only differences in outcomes observed between the three groups were in operation time, EBL and LOS. Median operation time was shortest in the proctor group and about 47 min shorter than in the training cases. This finding is comparable to outcomes of previous studies for training in laparoscopic colorectal surgery [36,37,38,39]. Although EBL was statistically significantly different between the three groups (training vs. graduate vs. proctor: 50, 50, 20 ml; p < 0.001), this is probably not clinically relevant and did not translate into a higher rate of post-operative complications.
Interestingly, LOS was different between the three cohorts, with the training cases being discharged one day later than the proctor and graduate cases. There are several factors that could have led to the observed results, in addition to the higher EBL observed in the training groups. Firstly, there were a higher number of benign resections in the graduate and training groups. These cases often include inflammatory conditions such as inflammatory bowel disease (IBD) or diverticulitis, which often take longer to recover from due to associated inflammatory pathology. Secondly, there are a larger number of abdominoperineal excisions (APERs) in the training group compared to the proctor group, which often have a more complex recovery. Thirdly, the difference in LOS could be due to differences in the discharge criteria of the 26 different centres across the 13 different countries, with some institutions behaving more proactively to discharge patients as early as possible due to associated bed pressures. However, it should be noted that the difference in LOS could be due to associated unaccounted surgical morbidity, which if higher in the training cohort could lead to a longer LOS. In two studies published by the Cleveland clinic group [40, 41], it was concluded that LOS, readmission rate and mortality can predict surgical morbidity in colorectal resections. Considering that our mortality and readmission rates are similar between groups, the difference in LOS could be attributed to associated morbidity.
A subgroup analysis of rectal resections yielded similar results to those of the overall cohort. Baseline characteristics, procedures performed and short-term outcomes were similar between the three groups, with the exception of operation time, EBL and LOS. This demonstrates that the conclusions drawn from the results of the overall examined cohort can be applied to robotic rectal resection surgery.
As far as we are aware there are only three previous studies describing robotic colorectal surgery training programmes [18, 34, 35]. The study by Winder et al. [34] offers a comprehensive description of a robotic training programme but includes a mix of general surgical cases, does not state how many colorectal resections were included and does not offer any description of surgical outcomes during the training process. Therefore, the study’s conclusions cannot be considered when discussing robotic colorectal surgery training pathways. The second study by Bell et al. [35] describes the robotic colorectal surgery training pathway of four surgeons in an academic hospital and presents the surgical outcomes of the surgeries performed (n = 48) during the training pathway. This study is descriptive and offers no comparison of outcomes for cases performed during or after training. The third study [18] was the first published study to report data from EARCS and included 82 robotic rectal resections performed by three EARCS graduates from two different centres, 30 of which were training cases. Similar to our study, there was no difference in the peri- or post-operative outcomes between the training and graduate groups with the exception of operative time, which was 36 min longer in the training cases.
To our knowledge, the presented study is the largest of its kind and is unique in comparing data between training cases, cases performed by delegates after they had completed their training and cases performed by proctors. Moreover, as far as we are aware this represents the first and largest European robotic colorectal surgery case series investigated to date, with over 1000 cases included from 26 different European centres.
Acknowledging our study limitations, there is obvious selection bias on the process of recruiting patients for proctorship, training and acquiring proficiency. Secondly, the database was derived from surgeon-reported data with its inherit risks of inadequate data entry and not all EARCS graduates submitted their data for this study. Additionally, there are differences in the proportion of benign cases between the three groups and there is a mixture of colorectal procedures included in this study with the proportion of those differing between the three investigated cohorts. However, a subgroup analysis of only rectal resections has demonstrated that the results are similar to those of the overall cohort and there are no baseline characteristic differences between the three groups for rectal resections. Moreover, multivariate regression analysis has mitigated for baseline characteristic differences and demonstrated that surgeon role (training vs. graduate vs. proctor) does not affect complication rates. Considering further limitations, although the large number of centres participating in this study greatly increases the external validity of the investigated outcomes, it makes it difficult to control potential confounding factors such as post-operative care, discharge criteria and robotic platform used, all of which could potentially lead to observation bias. However, considering all surgeons participated in the EARCS training programme, most surgeons applied relatively similar surgical techniques, therefore reducing surgical variability and operation-dependent observation bias. Finally, although data were collected from prospectively collated databases, the study was retrospective in nature and as a result there was no power calculation. However, this offers the advantage of the data being contemporary in nature—“real world” data—rather than data collected as part of a surgical trial that possibly includes an element of performance bias [42, 43]. In addition, all consecutive cases were included which minimises selection bias.
In conclusion, this study suggests that colorectal surgeons learning how to perform robotic surgery under the EARCS-structured training pathway can safely achieve short-term surgical outcomes comparable to their trainers and overcome the learning process in a way that minimises patient harm while achieving competency. Future studies including functional and long-term oncological data would be of great value in order to illuminate a more holistic comparison and strengthen the conclusions of this study.
Change history
07 January 2021
This article was updated to include Supplementary Information (the EARCS curriculum) and to correct the alignment of text in the PDF of Table 1, 2, and 6.
References
Weber PA, Merola S, Wasielewski A, Ballantyne GH (2002) Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 45:1686–1689. https://doi.org/10.1097/01.DCR.0000037657.78153.A8
AlAsari S, Min BS (2012) Robotic colorectal surgery: a systematic review. ISRN Surg 2012:1–12. https://doi.org/10.5402/2012/293894
Araujo SEA, Seid VE, Klajner S (2014) Robotic surgery for rectal cancer: current immediate clinical and oncological outcomes. World J Gastroenterol 20:14359–14370. https://doi.org/10.3748/wjg.v20.i39.14359
Mak TWC, Lee JFY, Futaba K, Hon SSF, Ngo DKY, Ng SSM (2014) Robotic surgery for rectal cancer: a systematic review of current practice. World J Gastrointest Oncol 6:184–193. https://doi.org/10.4251/wjgo.v6.i6.184
Surgical Intuitive (2017) da Vinci Products FAQ. https://isrg.gcs-web.com. Accessed 2 Aug 2019
Desouza AL, Prasad LM, Marecik SJ, Blumetti J, Park JJ, Zimmern A, Abcarian H (2010) Total mesorectal excision for rectal cancer: the potential advantage of robotic assistance. Dis Colon Rectum 53:1611–1617. https://doi.org/10.1007/DCR.0b013e3181f22f1f
Xiong B, Ma L, Huang W, Zhao Q, Cheng Y, Liu J (2015) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis of eight studies. J Gastrointest Surg 19:516–526. https://doi.org/10.1007/s11605-014-2697-8
Zhang X, Wei Z, Bie M, Peng X, Chen C (2016) Robot-assisted versus laparoscopic-assisted surgery for colorectal cancer: a meta-analysis. Surg Endosc 30:5601–5614. https://doi.org/10.1007/s00464-016-4892-z
Liao G, Zhao Z, Lin S, Li R, Yuan Y, Du S, Chen J, Deng H (2014) Robotic-assisted versus laparoscopic colorectal surgery: a meta-analysis of four randomized controlled trials. World J Surg Oncol 12:122. https://doi.org/10.1186/1477-7819-12-122
Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, Gulla N, Noya G, Boselli C, Gullà N, Noya G, Boselli C (2012) Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Color Dis 14:e134–e156. https://doi.org/10.1111/j.1463-1318.2011.02907.x
Lee SH, Lim S, Kim JH, Lee KY (2015) Robotic versus conventional laparoscopic surgery for rectal cancer: systematic review and meta-analysis. Ann Surg Treat Res 89:190–201. https://doi.org/10.4174/astr.2015.89.4.190
Xiong B, Ma L, Zhang C, Cheng Y (2014) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Surg Res 188:404–414. https://doi.org/10.1016/j.jss.2014.01.027
Scarpinata R, Aly EH (2013) Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum 56:253–262. https://doi.org/10.1097/DCR.0b013e3182694595
Wang Y, Zhao G-H, Yang H, Lin J (2016) A pooled analysis of robotic versus laparoscopic surgery for total mesorectal excision for rectal cancer. Surg Laparosc Endosc Percutan Tech 26:259–264. https://doi.org/10.1097/SLE.0000000000000263
Li X, Wang T, Yao L, Hu L, Jin P, Guo T, Yang K (2017) The safety and effectiveness of robot-assisted versus laparoscopic TME in patients with rectal cancer: a meta-analysis and systematic review. Medicine (Baltimore) 96:e7585. https://doi.org/10.1097/MD.0000000000007585
Sun Y, Xu H, Li Z, Han J, Song W, Wang J, Xu Z (2016) Robotic versus laparoscopic low anterior resection for rectal cancer: a meta-analysis. World J Surg Oncol 14:61. https://doi.org/10.1186/s12957-016-0816-6
Panteleimonitis S, Pickering O, Ahmad M, Harper M, Qureshi T, Figueiredo N, Parvaiz A (2019) Robotic rectal cancer surgery: results from a European multicentre case series of 240 resections and comparative analysis between cases performed with the da Vinci Si and Xi systems. Laparosc Endosc Robot Surg. https://doi.org/10.1016/J.LERS.2019.12.002
Panteleimonitis S, Popeskou S, Aradaib M, Harper M, Ahmed J, Ahmad M, Qureshi T, Figueiredo N, Parvaiz A (2018) Implementation of robotic rectal surgery training programme: importance of standardisation and structured training. Langenbeck’s Arch Surg. https://doi.org/10.1007/s00423-018-1690-1
Mackenzie H, Miskovic D, Ni M, Tan WS, Coleman MG, Hanna GB (2015) Risk prediction score in laparoscopic colorectal surgery training: experience from the english national training program. Ann Surg 261:338–344. https://doi.org/10.1097/SLA.0000000000000651
Coleman MG, Hanna GB, Kennedy R (2011) The national training programme for laparoscopic colorectal surgery in England: a new training paradigm. Colorectal Dis 13:614–616. https://doi.org/10.1111/j.1463-1318.2011.02643.x
EARCS (2019) European Academy of Robotic Colorectal Surgery graduates. https://earcs.pt/graduates.html. Accessed 2 Aug 2019
Kehlet H, Wilmore DW (2002) Multimodal strategies to improve surgical outcome. Am J Surg 183:630–641
Panteleimonitis S, Harper M, Hall S, Figueiredo N, Qureshi T, Parvaiz A (2017) Precision in robotic rectal surgery using the da Vinci Xi system and integrated table motion, a technical note. J Robot Surg. https://doi.org/10.1007/s11701-017-0752-7
Ahmed J, Siddiqi N, Khan L, Kuzu A, Parvaiz A (2016) Standardized technique for single-docking robotic rectal surgery. Color Dis 18:O380–O384. https://doi.org/10.1111/codi.13466
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Bolsin S, Colson M (2000) The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Heal care J Int Soc Qual Heal Care 12:433–438
Chang WR, McLean IP (2006) CUSUM: a tool for early feedback about performance? BMC Med Res Methodol 6:8. https://doi.org/10.1186/1471-2288-6-8
Carpenter BT, Sundaram CP (2017) Training the next generation of surgeons in robotic surgery. Robot Surg 4:39–44. https://doi.org/10.2147/RSRR.S70552
Fong Y, Buell JF, Collins J, Martinie J, Bruns C, Tsung A, Clavien P-A, Nachmany I, Edwin B, Pratschke J, Solomonov E, Koenigsrainer A, Giulianotti PC (2020) Applying the Delphi process for development of a hepatopancreaticobiliary robotic surgery training curriculum. Surg Endosc 34:4233–4244. https://doi.org/10.1007/s00464-020-07836-6
Huffman EM, Rosen SA, Levy JS, Martino MA, Stefanidis D (2020) Are current credentialing requirements for robotic surgery adequate to ensure surgeon proficiency? Surg Endosc. https://doi.org/10.1007/s00464-020-07608-2
MacCraith E, Forde JC, Davis NF (2019) Robotic simulation training for urological trainees: a comprehensive review on cost, merits and challenges. J Robot Surg 13:371–377. https://doi.org/10.1007/s11701-019-00934-1
Brook NR, Dell’Oglio P, Barod R, Collins J, Mottrie A (2019) Comprehensive training in robotic surgery. Curr Opin Urol 29:1–9. https://doi.org/10.1097/MOU.0000000000000566
Sridhar AN, Briggs TP, Kelly JD, Nathan S (2017) Training in robotic surgery—an overview. Curr Urol Rep 18:58. https://doi.org/10.1007/s11934-017-0710-y
Winder JS, Juza RM, Sasaki J, Rogers AM, Pauli EM, Haluck RS, Estes SJ, Lyn-Sue JR (2016) Implementing a robotics curriculum at an academic general surgery training program: our initial experience. J Robot Surg 10:209–213. https://doi.org/10.1007/s11701-016-0569-9
Bell S, Carne P, Chin M, Farmer C (2015) Establishing a robotic colorectal surgery programme. ANZ J Surg 85:214–216. https://doi.org/10.1111/ans.12817
De’Ath HD, Devoto L, Mehta C, Bromilow J, Qureshi T (2017) Mentored trainees have similar short-term outcomes to a consultant trainer following laparoscopic colorectal resection. World J Surg 41:1896–1902. https://doi.org/10.1007/s00268-017-3925-7
Langhoff PK, Schultz M, Harvald T, Rosenberg J (2013) Safe laparoscopic colorectal surgery performed by trainees. J Surg Educ 70:144–148. https://doi.org/10.1016/j.jsurg.2012.06.027
Daetwiler S, Guller U, Schob O, Adamina M (2007) Early introduction of laparoscopic sigmoid colectomy during residency. Br J Surg 94:634–641. https://doi.org/10.1002/bjs.5638
Dalton SJ, Ghosh AJ, Zafar N, Riyad K, Dixon AR (2010) Competency in laparoscopic colorectal surgery is achievable with appropriate training but takes time: a comparison of 300 elective resections with anastomosis. Colorectal Dis 12:1099–1104. https://doi.org/10.1111/j.1463-1318.2009.01998.x
Crawshaw BP, Keller DS, Brady JT, Augestad KM, Schiltz NK, Koroukian SM, Navale SM, Steele SR, Delaney CP (2017) The HARM score for gastrointestinal surgery: application and validation of a novel, reliable and simple tool to measure surgical quality and outcomes. Am J Surg 213:575–578. https://doi.org/10.1016/j.amjsurg.2016.11.007
Keller DS, Chien H-L, Hashemi L, Senagore AJ, Delaney CP (2014) The HARM score: a novel, easy measure to evaluate quality and outcomes in colorectal surgery. Ann Surg 259:1119–1125. https://doi.org/10.1097/SLA.0b013e3182a6f45e
Bothwell LE, Greene JA, Podolsky SH, Jones DS (2016) Assessing the gold standard-lessons from the history of RCTs. N Engl J Med 374:2175–2181. https://doi.org/10.1056/NEJMms1604593
McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D (2002) Randomised trials in surgery: problems and possible solutions. BMJ 324:1448–1451. https://doi.org/10.1136/bmj.324.7351.1448
Acknowledgements
We thank all participating centres registered with the EARCS collaborative for inputting and updating their data. EARCS Collaborators: Mukhtar Ahmad, Jamil Ahmed, Sergio Alfieri, Safaa Al-Ubaidi, Shwan Amin, Per Andersen, James Anderson, Heiko Aselmann, Thomas Bachleitner-Hoffmann, Thomas Becker, Paolo Bellora, Michael Bergmann, Paolo Pietro Bianchi, Rogier Crolla, Bernhard Dauser, Nicola de’Angelis, Jan Egberts, Charles Evans, Roger Gerjy, Marcos Gómez Ruiz, Adnan Hafeez, Mick Harper, Henrik Loft Jakobson, David Jayne, Mohammad Kalbassi, Christian Krieglstein, Yakup Kulu, Ayhan Kuzu, Per-Anders Larsson, Fabrizio Luca, Benno Mann, Daniel Mittmann, Beat Müller, Catharina Müller, Paul Neary, Maziar Nikberg, Diarmuid O’Riordain, Daniel Perez, Peiman Poornoroozy, Fabio Priora, Tahseen Qureshi, Tero Rautio, Philippe Rouanet, Andreas Scheiwiller, Irshad Shaikh, Matej Škrovina, Anke Smits, Niels Thomassen, Liviu Titu, Alain Valverde and George Van der Schelling.
Funding
No funding was received to produce or support this study.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosures
The infrastructure for central coordination of the European Academy of Robotic Colorectal Surgery (EARCS) is hosted by the Champalimaud Foundation (Avenida Brasilia, Lisbon, Portugal) with the previous support of Intuitive Surgical Sàrl (Chemin des Mûriers, 1, CH 1170 Aubonne, Switzerland). However, EARCS is a standalone project and central coordination is hosted at the Champalimaud Foundation, Avenida da Brasilia, 1400–038 Lisbon, Portugal. Some of the surgeons who participated in the study are proctors for Intuitive Surgical Sàrl. However, no surgeon was paid to participate in the study carried out as part of this paper. Sofoklis Panteleimonitis, Nuno Figueiredo, Rachelle Bissett-Amess, Matthias Turina and Richard J. Heald have no conflicts of interest or financial ties to disclose. Danilo Miskovic, Giuseppe Spinoglio and Amjad Parvaiz are proctors for Intuitive Surgical Sàrl.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was updated to include Supplementary Information (the EARCS curriculum) and to correct the alignment of text in Tables 1, 2, and 6.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Panteleimonitis, S., Miskovic, D., Bissett-Amess, R. et al. Short-term clinical outcomes of a European training programme for robotic colorectal surgery. Surg Endosc 35, 6796–6806 (2021). https://doi.org/10.1007/s00464-020-08184-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08184-1