Abstract
Background
Very few studies have evaluated the effectiveness of oral proton-pump inhibitors for the prevention of bleeding after endoscopic submucosal dissection (ESD) for gastric tumors. The aim of our study was to establish the non-inferiority of lansoprazole orally disintegrating (OD) tablets to intravenous lansoprazole for the prevention of bleeding from artificial ulcers after ESD.
Patients and methods
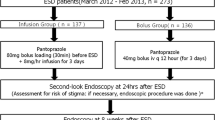
Consecutive patients who underwent ESD for gastric tumors were randomly assigned to receive lansoprazole OD tablets (OD group) or intravenous lansoprazole (IV group). In the OD group, lansoprazole OD tablets (30 mg) were given orally once daily for 8 weeks (56 days), starting on the day before ESD. In the IV group, lansoprazole (30 mg) was given as a continuous intravenous infusion twice daily for 3 days, starting on the day before ESD, and lansoprazole OD tablets (30 mg) were given orally once daily on days 4–56. The primary endpoint was the incidence of bleeding events within 8 weeks after ESD.
Results
Among 310 enrolled patients, 304 patients (152 in the OD group and 152 in the IV group) were included in the analysis. Endoscopic hemostasis was performed in 38 patients (19 in the OD group and 19 in the IV group). The incidence of bleeding events within 8 weeks after ESD did not differ significantly between the groups (p = 0.487). Endoscopic hemostasis was performed at second-look endoscopy in 17 patients (11.2%) in the OD group and 19 patients (12.5%) in the IV group (difference, 1.3 percentage points; 90% confidence interval, − 4.8–7.4%; non-inferiority, p < 0.001).
Conclusions
The effectiveness of lansoprazole OD tablets for the prevention of bleeding from artificial ulcers after ESD was similar to that of intravenous lansoprazole. Lansoprazole OD tablets are thus considered a treatment option in patients who undergo ESD.



Similar content being viewed by others
References
Ono H, Kondo H, Gotoda T et al (2001) Endoscopic mucosal resection for treatment of early gastric cancer. Gut 45:225–229
Nakamoto S, Sakai Y, Kasanuki J et al (2009) Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 41:746–750
Yamaguchi Y, Katsumi N, Aoki K et al (2007) Resection area of 15 mm as dividing line for choosing strip biopsy or endoscopic submucosal dissection for mucosal gastric neoplasm. J Clin Gastroenterol 41:472–476
Gotoda T, Yanagisawa A, Sasako M et al (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3:219–225
Tanabe S, Ishido K, Higuchi K et al (2014) Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer 17:130–136
Kakushima N, Yahagi N, Fujishiro M et al (2004) The healing process of gastric artificial ulcer after endoscopic submucosal dissection. Dig Endosc 16:327–331
Uedo N, Takeuchi Y, Yamada T et al (2007) Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 102:1610–1616
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Forrest JA, Finlayson ND, Shearman DJ (1974) Endoscopy in gastrointestinal bleeding. Lancet 17:394–397
Kazuei O (2005) The management of anticoagulation and antiplatelet therapy for endoscopic procedures. Gastroenterol Endosc 47:2691–2695 (Japanese).
National Cancer Institute. Common terminology criteria for adverse events (CTCAE) ver4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 20 Nov 2017
Ohkuwa M, Hosokawa K, Boku N et al (2001) New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy 33:221–226
Ono H, Hasuike N, Inui T et al (2008) Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 11:47–52
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Dunnett CW, Gent M (1977) Significance testing to establish equivalence between treatment, with special reference to data in the form of 2 × 2 tables. Biometrics 33:593–602
Dunnett CW, Gent M (1996) An alternative to the use of two-sided tests in clinical trials. Stat Med 15:1729–1738
Yang Z, Wu Q, Liu Z et al (2011) Proton pump inhibitors versus histamine-2-receptor antagonists for the management of iatrogenic gastric ulcer after endoscopic mucosal resection or endoscopic submucosal dissection: a meta-analysis of randomized trials. Digestion 84:315–320
Sachs G, Shin JM, Munson K et al (2000) Review article: the control of gastric acid and Helicobacter pylori eradication. Aliment Pharmacol Ther 14:1383–1401
Gisbert JP, González L, Calvet X et al (2001) Proton pump inhibitors versus H2-antagonists: a meta-analysis of their efficacy in treating bleeding peptic ulcer. Aliment Pharmacol Ther 15:917–926
Arikawa Y, Nishida H, Kurasawa O (2012) eta l. Discovery of a novel pyrrole derivative 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438) as a potassium-competitive acid blocker (P-CAB). J Med Chem 55:4446–4456
Jenkins H, Sakurai Y, Nishimura A et al (2015) Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 41:636–648
Takahashi K, Sato Y, Kohisa J et al (2016) vonoprazan 20 mg vs lansoprazole 30 mg for endoscopic submucosal dissection-induced gastric ulcers. World J Gastrointest Endosc 8:716–722
Tsuchiya I, Kato Y, Tanida E et al. Effect of vonoprazan on the treatment of artificial gastric ulcers after endoscopic submucosal dissection: a prospective randomized controlled trial. Dig Endosc. https://doi.org/10.1111/den.12857
Kubota T, Chiba K, Ishizaki T (1996) Genotyping of S-mephenytoin 4′-hydroxylation in an extended Japanese population. Clin Pharmacol Ther 60:661–666
Adachi K, Katsube T, Kawamura A et al (2000) CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole. Aliment Pharmacol Ther 14:1259–1266
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Kenji Ishido, Satoshi Tanabe, Mizutomo Azuma, Chikatoshi Katada, Takuya Wada, Takafumi Yano, and Wasaburo Koizumi have no conflicts of interest or financial ties to disclose.
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki. The protocol was approved by the institutional review board of the participating hospital.
Rights and permissions
About this article
Cite this article
Ishido, K., Tanabe, S., Azuma, M. et al. Comparison of oral and intravenous lansoprazole for the prevention of bleeding from artificial ulcers after endoscopic submucosal dissection for gastric tumors: a prospective randomized phase II study (KDOG 0802). Surg Endosc 32, 2939–2947 (2018). https://doi.org/10.1007/s00464-017-6008-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-6008-9