Abstract
The process dynamics of anaerobic oxidation of methane (AOM) coupled to sulfate reduction (SR), and the potential role of elemental sulfur as intermediate are presented in this paper. Thermodynamic screening and experimental evidence from the literature conclude that a prominent model to describe AOM-SR is based on the concept that anaerobic methane oxidation proceeds through the production of the intermediate elemental sulfur. Two microbial groups are involved in the process: (a) anaerobic methanotrophs (ANME-2) and (b) Desulfosarcina/Desulfococcus sulfur reducers cluster (DSS). In this work, a dynamic model was developed to explore the interactions between biotic and abiotic processes to simulate the microbial activity, the chemical composition and speciation of the liquid phase, and the gas phase composition in the reactor headspace. The model includes the microbial kinetics for the symbiotic growth of ANME-2 and DSS, mass transfer phenomena between the gas and liquid phase for methane, hydrogen sulfide, and carbon dioxide and acid–base reactions for bicarbonate, sulfide, and ammonium. A data set from batch experiments, running for 250 days in artificial seawater inoculated with sediment from Marine Lake Grevelingen (The Netherlands) was used to calibrate the model. The inherent characteristics of AOM-SR make the identification of the kinetic parameters difficult due to the high correlation between them. However, by meaningfully selecting a set of kinetic parameters, the model simulates successfully the experimental data for sulfate reduction and sulfide production. The model can be considered as the basic structure for simulating continuous flow three-phase engineered systems based on AOM-SR.


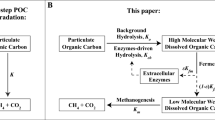
Reprinted with permission from [39]. Copyright (2007) American Chemical Society





Similar content being viewed by others
References
Bertrand J-C, Caumette P, Lebaron P, Matheron R, Normand P, Sime-Ngando T (2015) Environmental microbiology: fundamentals and applications. Springer, London
Jørgensen BB, Findlay AJ, Pellerin A (2019) The biogeochemical sulfur cycle of marine sediments. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00849
Knittel K, Boetius A, Lemke A, Eilers H, Lochte K, Pfannkuche O, Linke P, Amann R (2003) Activity, distribution, and diversity of sulfate reducers and other bacteria in sediments above gas hydrate (Cascadia Margin, Oregon). Geomicrobiol J 20(4):269–294. https://doi.org/10.1080/01490450303896
Knittel K, Boetius A (2011) Anaerobic oxidation of methane with sulfate. In: Reitner J, Thiel V (eds) Encyclopedia of geobiology. Springer, Netherlands, Dordrecht, pp 36–47. https://doi.org/10.1007/978-1-4020-9212-1_10
Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, Gieseke A, Amann R, Jørgensen BB, Witte U, Pfannkuche O (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407(6804):623–626. https://doi.org/10.1038/35036572
Devol AH, Anderson JJ, Kuivila K, Murray JW (1984) A model for coupled sulfate reduction and methane oxidation in the sediments of Saanich Inlet. Geochim Cosmochim Acta 48(5):993–1004. https://doi.org/10.1016/0016-7037(84)90191-1
Iversen N, Jorgensen BB (1985) Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark) 1. Limnol Oceanogr 30(5):944–955. https://doi.org/10.4319/lo.1985.30.5.0944
Milucka J, Ferdelman TG, Polerecky L, Franzke D, Wegener G, Schmid M, Lieberwirth I, Wagner M, Widdel F, Kuypers MMM (2012) Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 491:541. https://doi.org/10.1038/nature11656
Alperin M, Reeburgh W, Whiticar M (1988) Carbon and hydrogen isotope fractionation resulting from anaerobic methane oxidation. Global Biogeochem Cycles 2(3):279–288. https://doi.org/10.1029/GB002i003p00279
Holler T, Wegener G, Knittel K, Boetius A, Brunner B, Kuypers MMM, Widdel F (2009) Substantial 13C/12C and D/H fractionation during anaerobic oxidation of methane by marine consortia enriched in vitro. Environ Microbiol Rep 1(5):370–376. https://doi.org/10.1111/j.1758-2229.2009.00074.x
McGlynn SE, Chadwick GL, Kempes CP, Orphan VJ (2015) Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature 526:531. https://doi.org/10.1038/nature15512
Musat N, Musat F, Weber PK, Pett-Ridge J (2016) Tracking microbial interactions with NanoSIMS. Curr Opin Biotechnol 41:114–121. https://doi.org/10.1016/j.copbio.2016.06.007
Haroon MF, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan Z, Tyson GW (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500(7464):567–570. https://doi.org/10.1038/nature12375
Wegener G, Krukenberg V, Riedel D, Tegetmeyer HE, Boetius A (2015) Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 526(7574):587–590. https://doi.org/10.1038/nature15733
Rinke C, Lee J, Nath N, Goudeau D, Thompson B, Poulton N, Dmitrieff E, Malmstrom R, Stepanauskas R, Woyke T (2014) Obtaining genomes from uncultivated environmental microorganisms using FACS–based single-cell genomics. Nat Protoc 9(5):1038–1048. https://doi.org/10.1038/nprot.2014.067
Dufrêne YF (2014) Atomic force microscopy in microbiology: new structural and functional insights into the microbial cell surface. mBiol 5(4):e01363-01314. https://doi.org/10.1128/mBio.01363-14
Hoehler TM, Alperin MJ, Albert DB, Martens CS (1994) Field and laboratory studies of methane oxidation in an anoxic marine sediment: Evidence for a methanogen-sulfate reducer consortium. Global Biogeochem Cycles 8(4):451–463. https://doi.org/10.1029/94gb01800
Bhattarai S, Cassarini C, Rene ER, Zhang Y, Esposito G, Lens PNL (2018) Enrichment of sulfate reducing anaerobic methane oxidizing community dominated by ANME-1 from Ginsburg Mud Volcano (Gulf of Cadiz) sediment in a biotrickling filter. Bioresour Technol 259:433–441. https://doi.org/10.1016/j.biortech.2018.03.018
Bhattarai S, Cassarini C, Naangmenyele Z, Rene ER, Gonzalez-Gil G, Esposito G, Lens PNL (2017) Microbial sulfate-reducing activities in anoxic sediment from Marine Lake Grevelingen: screening of electron donors and acceptors. Limnology 19(1):31–41. https://doi.org/10.1007/s10201-017-0516-0
Meulepas RJW, Jagersma CG, Gieteling J, Buisman CJN, Stams AJM, Lens PNL (2009) Enrichment of anaerobic methanotrophs in sulfate-reducing membrane bioreactors. Biotechnol Bioeng 104(3):458–470. https://doi.org/10.1002/bit.22412
Cassarini C, Rene ER, Bhattarai S, Vogt C, Musat N, Lens PNL (2019) Anaerobic methane oxidation coupled to sulfate reduction in a biotrickling filter: Reactor performance and microbial community analysis. Chemosphere 236:124290. https://doi.org/10.1016/j.chemosphere.2019.07.021
Amodeo C, Masi S, Van Hulle SWH, Zirpoli P, Mancini IM, Caniani D (2015) Methane oxidation in a biofilter (Part 1): development of a mathematical model for designing and optimization. J Environ Sci Health Part A 50(13):1393–1403. https://doi.org/10.1080/10934529.2015.1064277
Amodeo C, Masi S, Van Hulle SWH, Zirpoli P, Mancini IM, Caniani D (2015) Methane oxidation in a biofilter (Part 2): A lab-scale experiment for model calibration. J Environ Sci Health Part A 50(13):1404–1409. https://doi.org/10.1080/10934529.2015.1064278
He Z, Cai C, Geng S, Lou L, Xu X, Zheng P, Hu B (2013) Modelling a nitrite-dependent anaerobic methane oxidation process: parameters identification and model evaluation. Bioresour Technol 147:315–320. https://doi.org/10.1016/j.biortech.2013.08.001
Modin O (2018) A mathematical model of aerobic methane oxidation coupled to denitrification. Environ Technol 39(9):1217–1225. https://doi.org/10.1080/09593330.2017.1323961
Lee H-S, Tang Y, Rittmann BE, Zhao H-P (2018) Anaerobic oxidation of methane coupled to denitrification: fundamentals, challenges, and potential. Crit Rev Environ Sci Technol 48(19–21):1067–1093. https://doi.org/10.1080/10643389.2018.1503927
Vavilin VA (2018) Anaerobic methane oxidation by nitrate: kinetic isotope effect. Environ Dyn Glob Clim Change 10 (1):3–16. Doi: https://doi.org/10.17816/edgcc10534
He X, Chadwick G, Kempes C, Shi Y, McGlynn S, Orphan V, Meile C (2019) Microbial interactions in the anaerobic oxidation of methane: model simulations constrained by process rates and activity patterns. Environ Microbiol 21(2):631–647. https://doi.org/10.1111/1462-2920.14507
Butler JN (1991) Carbon dioxide equilibria and their applications. Lewis Publishers, Michigan
Barnes R, Goldberg E (1976) Methane production and consumption in anoxic marine sediments. Geology 4(5):297–300. https://doi.org/10.1130/0091-7613(1976)4%3C297:MPACIA%3E2.0.CO;2
Boudreau BP (1997) Diagenetic models and their implementation, vol 505. Springer, Berlin
Egger M, Lenstra W, Jong D, Meysman FJ, Sapart CJ, Van der Veen C, Röckmann T, Gonzalez S, Slomp CP (2016) Rapid sediment accumulation results in high methane effluxes from coastal sediments. PLoS ONE 11(8):1–22. https://doi.org/10.1371/journal.pone.0161609
Rooze J, Egger M, Tsandev I, Slomp CP (2016) Iron-dependent anaerobic oxidation of methane in coastal surface sediments: potential controls and impact. Limnol Oceanogr 61(S1):S267–S282. https://doi.org/10.1002/lno.10275
Meulepas RJW, Stams AJM, Lens PNL (2010) Biotechnological aspects of sulfate reduction with methane as electron donor. Rev Environ Sci Bio/Technol 9(1):59–78. https://doi.org/10.1007/s11157-010-9193-8
Bhattarai S, Cassarini C, Lens PNL (2019) Physiology and distribution of archaeal methanotrophs that couple anaerobic oxidation of methane with sulfate reduction. Microbiol Mol Biol Rev. https://doi.org/10.1128/MMBR.00074-18
Valentine DL, Reeburgh WS (2000) New perspectives on anaerobic methane oxidation. Environ Microbiol 2(5):477–484. https://doi.org/10.1046/j.1462-2920.2000.00135.x
Hinrichs K-U, Boetius A (2002) The anaerobic oxidation of methane: new insights in microbial ecology and biogeochemistry. In: Wefer G, Billett D, Hebbeln D, Jørgensen BB, Schlüter M, van Weering TCE (eds) Ocean margin systems. Springer Berlin Heidelberg, Berlin, pp 457–477. https://doi.org/10.1007/978-3-662-05127-6_28
Alperin MJ, Hoehler TM (2009) Anaerobic methane oxidation by archaea/sulfate-reducing bacteria aggregates: 1. Thermodynamic and physical constraints. Am J Sci 309(10):869–957. https://doi.org/10.2475/10.2009.01
Rickard D, Luther GW (2007) Chemistry of iron sulfides. Chem Rev 107(2):514–562. https://doi.org/10.1021/cr0503658
Rittmann BE, McCarty PL (2020) Environmental biotechnology: principles and applications, 2nd edn. McGraw-Hill, New York
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41(1):100–180
Dolfing J, Janssen DB (1994) Estimates of Gibbs free energies of formation of chlorinated aliphatic compounds. Biodegradation 5(1):21–28. https://doi.org/10.1007/BF00695210
Dean JA (1999) Lange’s handbook of chemistry, 15th edn. McGraw-Hill, New York
Amend JP, Shock EL (2001) Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and bacteria. FEMS Microbiol Rev 25(2):175–243. https://doi.org/10.1111/j.1574-6976.2001.tb00576.x
Stams AJM, Plugge CM (2009) Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat Rev Microbiol 7(8):568–577. https://doi.org/10.1038/nrmicro2166
Sorensen KB, Finster K, Ramsing NB (2001) Thermodynamic and kinetic requirements in anaerobic methane oxidizing consortia exclude hydrogen, acetate, and methanol as possible electron shuttles. Microb Ecol 42(1):1–10. https://doi.org/10.1007/s002480000083
Orcutt B, Meile C (2008) Constraints on mechanisms and rates of anaerobic oxidation of methane by microbial consortia: process-based modeling of ANME-2 archaea and sulfate reducing bacteria interactions. Biogeosciences 5 (6):1587-1599. https://doi.org/10.5194/bg-5-1587-2008
Nauhaus K, Boetius A, Krüger M, Widdel F (2002) In vitro demonstration of anaerobic oxidation of methane coupled to sulphate reduction in sediment from a marine gas hydrate area. Environ Microbiol 4(5):296–305. https://doi.org/10.1046/j.1462-2920.2002.00299.x
Kamyshny A, Gun J, Rizkov D, Voitsekovski T, Lev O (2007) Equilibrium distribution of polysulfide ions in aqueous solutions at different temperatures by rapid single phase derivatization. Environ Sci Technol 41(7):2395–2400. https://doi.org/10.1021/es062637+
Helz GR (2014) Activity of zero-valent sulfur in sulfidic natural waters. Geochem Trans 15:13–13. https://doi.org/10.1186/s12932-014-0013-x
Kleinjan WE, de Keizer A, Janssen AJH (2005) Equilibrium of the reaction between dissolved sodium sulfide and biologically produced sulfur. Colloids Surf B Biointerfaces 43(3):228–237. https://doi.org/10.1016/j.colsurfb.2005.05.004
Kamyshny A, Goifman A, Gun J, Rizkov D, Lev O (2004) Equilibrium distribution of polysulfide ions in aqueous solutions at 25 degrees C: a new approach for the study of polysulfides’ equilibria. Environ Sci Technol 38(24):6633–6644. https://doi.org/10.1021/es049514e
Rickard D (2012) Sulfidic sediments and sedimentary rocks, Developments in sedimentology, vol 65. Elsevier, Amsterdam
Krämer M, Cypionka H (1989) Sulfate formation via ATP sulfurylase in thiosulfate- and sulfite-disproportionating bacteria. Arch Microbiol 151(3):232–237. https://doi.org/10.1007/bf00413135
Finster K, Liesack W, Thamdrup B (1998) Elemental sulfur and thiosulfate disproportionation by Desulfocapsa sulfoexigens sp. Nov., a new anaerobic bacterium isolated from marine surface sediment. Appl Environ Microbiol 64(1):119–125
Finster K (2008) Microbiological disproportionation of inorganic sulfur compounds. J Sulfur Chem 29(3–4):281–292. https://doi.org/10.1080/17415990802105770
Cassarini C, Rene ER, Bhattarai S, Esposito G, Lens PNL (2017) Anaerobic oxidation of methane coupled to thiosulfate reduction in a biotrickling filter. Bioresour Technol 240:214–222. https://doi.org/10.1016/j.biortech.2017.03.003
Vavilin V, Lokshina L, Rytov S (2019) Using kinetic isotope effect to evaluate the significance of the sequential and parallel steps: formation of microbial consortium during reversible anaerobic methane oxidation coupled with sulfate reduction. Water Sci Technol 79(11):2056–2067. https://doi.org/10.2166/wst.2019.201
McCarty PL (2007) Thermodynamic electron equivalents model for bacterial yield prediction: modifications and comparative evaluations. Biotechnol Bioeng 97(2):377–388. https://doi.org/10.1002/bit.21250
Dale AW, Regnier P, Van Cappellen P (2006) Bioenergetic controls on anaerobic oxidation of methane (AOM) in coastal marine sediments: a theoretical analysis. Am J Sci 306(4):246–294. https://doi.org/10.2475/ajs.306.4.246
Nauhaus K, Albrecht M, Elvert M, Boetius A, Widdel F (2007) In vitro cell growth of marine archaeal-bacterial consortia during anaerobic oxidation of methane with sulfate. Environ Microbiol 9(1):187–196. https://doi.org/10.1111/j.1462-2920.2006.01127.x
Zhang Y, Henriet J-P, Bursens J, Boon N (2010) Stimulation of in vitro anaerobic oxidation of methane rate in a continuous high-pressure bioreactor. Bioresour Technol 101(9):3132–3138. https://doi.org/10.1016/j.biortech.2009.11.103
Beyenal H, Chen SN, Lewandowski Z (2003) The double substrate growth kinetics of Pseudomonas aeruginosa. Enzyme Microb Technol 32(1):92–98. https://doi.org/10.1016/S0141-0229(02)00246-6
Zinn M, Witholt B, Egli T (2004) Dual nutrient limited growth: models, experimental observations, and applications. J Biotechnol 113(1–3):263–279. https://doi.org/10.1016/j.jbiotec.2004.03.030
Cherif M, Loreau M (2010) Towards a more biologically realistic use of Droop’s equations to model growth under multiple nutrient limitation. Oikos 119(6):897–907. https://doi.org/10.1111/j.1600-0706.2010.18397.x
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York, Chichester
Zeebe RE, Wolf-Gladrow D (2001) CO2 in seawater: equilibrium, kinetics, isotopes, vol 65. Elsevier, Amsterdam
Rumble JR, Lide DR, Bruno TJ (2018) CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. CRC Press, Boca Raton
Iversen N, Jørgensen BB (1993) Diffusion coefficients of sulfate and methane in marine sediments: Influence of porosity. Geochim Cosmochim Acta 57(3):571–578. https://doi.org/10.1016/0016-7037(93)90368-7
Poling BE, Prausnitz JM, O'Connell JP, Reid RCPog, liquids (2001) The properties of gases and liquids. 5th ed. / Bruce E. Poling, John M. Prausnitz, John P. O'Connell. edn. McGraw-Hill, New York ; London
Teramoto M, Tai S, Nishii K, Teranishi H (1974) Effects of pressure on liquid-phase mass transfer coefficients. Chem Eng J 8(3):223–226. https://doi.org/10.1016/0300-9467(74)85027-6
Sander R (2015) Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos Chem Phys 15(8):4399–4981
Girguis PR, Cozen AE, DeLong EF (2005) Growth and population dynamics of anaerobic methane-oxidizing archaea and sulfate-reducing bacteria in a continuous-flow bioreactor. Appl Environ Microbiol 71(7):3725–3733. https://doi.org/10.1128/aem.71.7.3725-3733.2005
Cassarini C, Zhang Y, Lens PNL (2019) Pressure selects dominant anaerobic methanotrophic phylotype and sulfate reducing bacteria in coastal marine lake Grevelingen sediment. Front Environ Sci. https://doi.org/10.3389/fenvs.2018.00162
Bhattarai S, Cassarini C, Gonzalez-Gil G, Egger M, Slomp CP, Zhang Y, Esposito G, Lens PNL (2017) Anaerobic methane-oxidizing microbial community in a coastal marine sediment: anaerobic methanotrophy dominated by ANME-3. Microb Ecol 74(3):608–622. https://doi.org/10.1007/s00248-017-0978-y
Caldwell SL, Laidler JR, Brewer EA, Eberly JO, Sandborgh SC, Colwell FS (2008) Anaerobic oxidation of methane: mechanisms, bioenergetics, and the ecology of associated microorganisms. Environ Sci Technol 42(18):6791–6799. https://doi.org/10.1021/es800120b
Valentine DL (2002) Biogeochemistry and microbial ecology of methane oxidation in anoxic environments: a review. Antonie Van Leeuwenhoek 81(1):271–282. https://doi.org/10.1023/A:1020587206351
Meyerdierks A, Kube M, Kostadinov I, Teeling H, Glöckner FO, Reinhardt R, Amann R (2010) Metagenome and mRNA expression analyses of anaerobic methanotrophic archaea of the ANME-1 group. Environ Microbiol 12(2):422–439. https://doi.org/10.1111/j.1462-2920.2009.02083.x
Moran JJ, Beal EJ, Vrentas JM, Orphan VJ, Freeman KH, House CH (2008) Methyl sulfides as intermediates in the anaerobic oxidation of methane. Environ Microbiol 10(1):162–173. https://doi.org/10.1111/j.1462-2920.2007.01441.x
Krukenberg V, Riedel D, Gruber-Vodicka HR, Buttigieg PL, Tegetmeyer HE, Boetius A, Wegener G (2018) Gene expression and ultrastructure of meso- and thermophilic methanotrophic consortia. Environ Microbiol 20(5):1651–1666. https://doi.org/10.1111/1462-2920.14077
Wang F-P, Zhang Y, Chen Y, He Y, Qi J, Hinrichs K-U, Zhang X-X, Xiao X, Boon N (2014) Methanotrophic archaea possessing diverging methane-oxidizing and electron-transporting pathways. ISME J 8(5):1069–1078. https://doi.org/10.1038/ismej.2013.212
Skennerton CT, Chourey K, Iyer R, Hettich RL, Tyson GW, Orphan VJ (2017) Methane-fueled syntrophy through extracellular electron transfer: uncovering the genomic traits conserved within diverse bacterial partners of anaerobic methanotrophic archaea. mBio 8(4):e00530-00517. https://doi.org/10.1128/mBio.00530-17
Gao Y, Lee J, Neufeld JD, Park J, Rittmann BE, Lee H-S (2017) Anaerobic oxidation of methane coupled with extracellular electron transfer to electrodes. Sci Rep 7(1):1–9. https://doi.org/10.1038/s41598-017-05180-9
Acknowledgements
The experimental part of this paper was funded by the Erasmus Mundus Joint Doctorate Programme ETeCoS3 (Environmental Technologies for Contaminated Solids, Soils and Sediments, Grant Agreement FPA No. 2010-0009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Code availability
The source code developed in this paper is available without support for non-commercial use upon request from the corresponding author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hatzikioseyian, A., Bhattarai, S., Cassarini, C. et al. Dynamic modeling of anaerobic methane oxidation coupled to sulfate reduction: role of elemental sulfur as intermediate. Bioprocess Biosyst Eng 44, 855–874 (2021). https://doi.org/10.1007/s00449-020-02495-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02495-2