Abstract
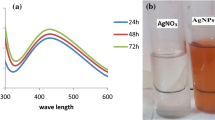
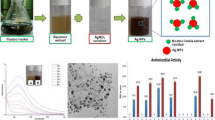
The present study reports the optimization of a green method for the synthesis of silver nanoparticles (AgNPs) via reduction of Ag+ ions using cell-free supernatant of mutant Bacillus licheniformis M09. UV–Visible spectroscopy showing an absorption peak at ~ 430 nm confirmed the synthesis of AgNPs. Transmission electron microscope (TEM) analysis exhibited spherical AgNPs within the size range of 10–30 nm. Fourier transform infrared (FTIR) measurements assured the presence of effective functional molecules which could be responsible for stabilizing the AgNPs. X-ray diffraction (XRD) pattern verified the crystalline nature of AgNPs. Furthermore, the synthesized AgNPs showed an excellent photocatalytic degradation of methylene blue dye in less than 3 h under visible light proving their potential as a catalytic agent for bioremediation for next-generation dye degradation in effluent treatment. The AgNPs demonstrated antimicrobial activity against Gram-positive and Gram-negative foodborne pathogens which endorsed its suitability as agents to extend shelf-life in food packaging and food safety applications. The results also revealed a strong concentration-dependent cytotoxicity of AgNPs against human breast adenocarcinoma cells (MCF-7), while 15.07 µg/mL of IC50 was attained. The outcome suggests the possible application of these AgNPs in nanomedicine formulations. Thus, these findings propose promising ways for the valorization of the waste fermentation supernatant left after cell harvesting and desired metabolite extraction.
Graphical abstract








Similar content being viewed by others
References
Vidor FF, Meyers T, Müller K et al (2017) Inverter circuits on freestanding flexible substrate using ZnO nanoparticles for cost-efficient electronics. Solid State Electron 137:16–21. https://doi.org/10.1016/j.sse.2017.07.011
Gittins DI, Bethell D, Nichols RJ, Schiffrin DJ (2000) Diode-like electron transfer across nanostructured films containing a redox ligand. J Mater Chem 10:79–83. https://doi.org/10.1039/a902960e
Crooks R, Lemon B III, Sun L et al (2001) Dendrimer-encapsulated metals and semiconductors: synthesis, characterization, and applications. Top Curr Chem 212:81–135
Chen S, Fu P, Yin B et al (2011) Immobilizing Pt nanoparticles and chitosan hybrid film on polyaniline nanofibers membrane for an amperometric hydrogen peroxide biosensor. Bioprocess Biosyst Eng 34:711–719. https://doi.org/10.1007/s00449-011-0520-4
Ladole MR, Nair RR, Bhutada YD et al (2018) Synergistic effect of ultrasonication and co-immobilized enzymes on tomato peels for lycopene extraction. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2018.06.013
Muley AB, Thorat AS, Singhal RS, Babu KH (2018) A tri-enzyme co-immobilized magnetic complex: Process details, kinetics, thermodynamics and applications. Int J Biol Macromol 118:1781–1795. https://doi.org/10.1016/j.ijbiomac.2018.07.022
Singh M, Chandrasekaran N, Mukherjee A et al (2014) Cancerous cell targeting and destruction using pH stabilized amperometric bioconjugated gold nanoparticles from marine macroalgae, Padina gymnospora. Bioprocess Biosyst Eng 37:1859–1869. https://doi.org/10.1007/s00449-014-1160-2
Jacob SJP, Prasad VLS, Sivasankar S, Muralidharan P (2017) Biosynthesis of silver nanoparticles using dried fruit extract of Ficus carica—screening for its anticancer activity and toxicity in animal models. Food Chem Toxicol 109:951–956. https://doi.org/10.1016/j.fct.2017.03.066
Konishi Y, Ohno K, Saitoh N et al (2007) Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol 128:648–653. https://doi.org/10.1016/j.jbiotec.2006.11.014
Willner I, Baron R, Willner B (2006) Growing metal nanoparticles by enzymes. Adv Mater 18:1109–1120. https://doi.org/10.1002/adma.200501865
Firdhouse MJ, Lalitha P (2015) Biosynthesis of silver nanoparticles and its applications. J Nanotechnol. https://doi.org/10.1155/2015/829526
Kumar A, Mandal S, Selvakannan PR et al (2003) Investigation into the interaction between surface-bound alkylamines and gold nanoparticles. Langmuir 19:6277–6282. https://doi.org/10.1021/la034209c
Malvindi MA, Di Corato R, Curcio A et al (2011) Multiple functionalization of fluorescent nanoparticles for specific biolabeling and drug delivery of dopamine. Nanoscale 3:5110–5119. https://doi.org/10.1039/c1nr10797f
Parashar UK, Saxena PS (2009) Bioinspired synthesis of silver nanoparticles. J Nanomater 4:159–166
Paul M, Pal N, Bhaumik A (2012) Selective adsorption and release of cationic organic dye molecules on mesoporous borosilicates. Mater Sci Eng C 32:1461–1468. https://doi.org/10.1016/j.msec.2012.04.026
Pal J, Deb MK (2014) Efficient adsorption of congo red dye from aqueous solution using green synthesized coinage nanoparticles coated activated carbon beads. Appl Nanosci 4:967–978. https://doi.org/10.1007/s13204-013-0277-y
Rosales E, Pérez-Paz A, Vázquez X et al (2012) Isolation of novel benzo[a]anthracene-degrading microorganisms and continuous bioremediation in an expanded-bed bioreactor. Bioprocess Biosyst Eng 35:851–855. https://doi.org/10.1007/s00449-011-0669-x
Gadd GM (2004) Microbial influence on metal mobility and application for bioremediation. Geoderma 122:109–119. https://doi.org/10.1016/j.geoderma.2004.01.002
Almeida É, De Oliveira D, Hotza D (2017) Characterization of silver nanoparticles produced by biosynthesis mediated by Fusarium oxysporum under different processing conditions. Bioprocess Biosyst Eng 40:1291–1303. https://doi.org/10.1007/s00449-017-1788-9
Jenzsch M, Simutis R, Eisbrenner G et al (2006) Estimation of biomass concentrations in fermentation processes for recombinant protein production. Bioprocess Biosyst Eng 29:19–27. https://doi.org/10.1007/s00449-006-0051-6
Momin B, Chakraborty S, Annapure U (2018) Investigation of the cell disruption methods for maximizing the extraction of arginase from mutant Bacillus licheniformis (M09) using statistical approach. Korean J Chem Eng 35:1–12. https://doi.org/10.1007/s11814-018-0107-8
Quinteros MA, Aiassa Martínez IM, Dalmasso PR, Páez PL (2016) Silver Nanoparticles: Biosynthesis using an ATCC reference strain of Pseudomonas aeruginosa and activity as broad spectrum clinical antibacterial agents. Int J Biomater. https://doi.org/10.1155/2016/5971047
Jeevan P, Ramya K, Rena AE (2012) Extracellular biosynthesis of silver nanoparticles by culture supernatant of Pseudomonas aeruginosa. Indian J Biotechnol 11:72–76
Philip D (2009) Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim Acta - Part A Mol Biomol Spectrosc 73:374–381. https://doi.org/10.1016/j.saa.2009.02.037
Huang J, Li Q, Sun D et al (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. https://doi.org/10.1088/0957-4484/18/10/105104
Sanghi R, Verma P (2009) Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour Technol 100:501–504. https://doi.org/10.1016/j.biortech.2008.05.048
Muley AB, Chaudhari SA, Mulchandani KH, Singhal RS (2018) Extraction and characterization of chitosan from prawn shell waste and its conjugation with cutinase for enhanced thermo-stability. Int J Biol Macromol 111:1047–1058. https://doi.org/10.1016/j.ijbiomac.2018.01.115
Ajitha B, Kumar YA, Reddy PS (2014) Molecular and Biomolecular Spectroscopy Biosynthesis of silver nanoparticles using Plectranthus amboinicus leaf extract and its antimicrobial activity. Spectrochim ACTA Part A Mol Biomol Spectrosc 128:257–262. https://doi.org/10.1016/j.saa.2014.02.105
Kishore Y, Sujit M, Behera K (2014) Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by Streptomyces sp. SS2:2263–2269. https://doi.org/10.1007/s00449-014-1205-6
Vanaja M, Gnanajobitha G, Paulkumar K et al (2013) Phytosynthesis of silver nanoparticles by Cissus quadrangularis: Influence of physicochemical factors. J Nanostruct Chem 3:17. https://doi.org/10.1186/2193-8865-3-17
Drzewiecka WW, Gaikwad S, Laskowski D, Niedojadło HD et al (2014) Novel approach towards synthesis of silver nanoparticles from Myxococcus virescens and their lethality on pathogenic bacterial cells. J Biotechnol Bioeng 1:1–7
Kalpana D, Lee YS (2013) Synthesis and characterization of bactericidal silver nanoparticles using cultural filtrate of simulated microgravity grown Klebsiella pneumoniae. Enzyme Microb Technol 52:151–156. https://doi.org/10.1016/j.enzmictec.2012.12.006
Vanaja M, Paulkumar K, Baburaja M et al (2014) Degradation of methylene blue using biologically synthesized silver nanoparticles. Bioinorg Chem Appl 2014:1–8
Zhu Y, Dan Y (2010) Photocatalytic activity of poly(3-hexylthiophene)/titanium dioxide composites for degrading methyl orange. Sol Energy Mater Sol Cells 94:1658–1664. https://doi.org/10.1016/j.solmat.2010.05.025
Liu Y, Xie S, Li H, Wang X (2014) A highly efficient sunlight driven ZnO nanosheet photocatalyst: synergetic effect of p-doping and MoS2 atomic layer loading. ChemCatChem 6:2522–2526. https://doi.org/10.1002/cctc.201402191
Yu L, Xi J, Li M-D et al (2012) The degradation mechanism of methyl orange under photo-catalysis of TiO2. Phys Chem Chem Phys 14:3589. https://doi.org/10.1039/c2cp23226j
Houas A (2001) Photocatalytic degradation pathway of methylene blue in water. Appl Catal B Environ 31:145–157. https://doi.org/10.1016/S0926-3373(00)00276-9
Ameta A, Ameta R, Ahuja M (2013) Photocatalytic degradation of methylene blue over ferric tungstate. Sci Rev Chem Commun 3:172–180
Borase HP, Patil CD, Salunkhe RB et al (2014) Transformation of aromatic dyes using green synthesized silver nanoparticles. Bioprocess Biosyst Eng 37:1695–1705. https://doi.org/10.1007/s00449-014-1142-4
Khan AU, Malik N, Khan M et al (2018) Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-017-1846-3
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11:371–384. https://doi.org/10.1038/nrmicro3028
Sudha A, Jeyakanthan J, Srinivasan P (2017) Resource-efficient technologies green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour Technol 3:506–515. https://doi.org/10.1016/j.reffit.2017.07.002
Gopinath V, Priyadarshini S, Fai M et al (2017) Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab J Chem 10:1107–1117. https://doi.org/10.1016/j.arabjc.2015.11.011
Acknowledgements
Authors are grateful to the University Grants Commission, India, for the financial support provided for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest towards this research.
Ethical approval
This article does not include any studies with human participants or animals performed by any authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Momin, B., Rahman, S., Jha, N. et al. Valorization of mutant Bacillus licheniformis M09 supernatant for green synthesis of silver nanoparticles: photocatalytic dye degradation, antibacterial activity, and cytotoxicity. Bioprocess Biosyst Eng 42, 541–553 (2019). https://doi.org/10.1007/s00449-018-2057-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-2057-2