Abstract
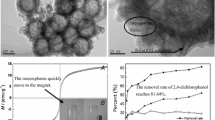
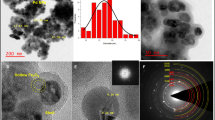
Functional porous hollow microspheres with superparamagnetism, Fe3O4/P(GMA–DVB–St) microspheres, were prepared via a dispersion polymerization based on hollow Fe3O4 microspheres. The resulting hollow microspheres were characterized by means of Fourier-transform infrared spectrophotometer (FT-IR), X-ray diffraction (XRD), transmission electron microscopy (TEM), Brunauer-Emmett-Teller (BET) gas sorptometry, and vibrating sample magnetometer (VSM). It is verified that the resulting hollow microspheres are porous and have high saturation magnetization. For further application, candida rugosa lipase (CRL) was immobilized onto the hollow microshperes, the loading amount of lipase was 143.88 mg CRL/g support and the activity recovery of the obtained immobilized lipase reached 73.25%. Besides, the resulting immobilized CRL (ICRL) were found to have better pH endurance and temperature endurance than the free ones, which showed the optimal catalytic activity with pH of 9.0 and temperature of 60 °C. The ICRL displayed excellent reusability as well.









Similar content being viewed by others
References
Gardossi L, Bianchi D, Klibanov AM(1991) Selective acylation of peptides catalyzed by lipases in organic solvents. J Am Chem Soc 113(16):6328–6329
Jaeger K, Reetz M (1998) Microbial lipases form versatile tools for biotechnology. Trends Biotechnol 16(9):396–403
Omprakash Y, Toyoko I (2005) Covalent-bonded immobilization of lipase on poly(phenylene sulfide) dendrimers and their hydrolysis ability. Biomacromolecules 6(5):2809–2814
Cowan DA, Fernandez-Lafuente R (2011) Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb Technol 49(4):326–346
Barriuso J, Vaquero ME, Prieto A, Martínez MJ (2016) Structural traits and catalytic versatility of the lipases from the Candida rugosa-like family: a review. Biothechnol Adv 34:874–885
Zivkovi LTI, Zivkovi LS, Beskoski VP, Gopcevi KR, Jokic BM, Radosavljevic DS, Karadzi IM (2016) The Candida rugosa lipase adsorbed onto titania as nano biocatalyst with improved thermostability and reuse potential in aqueous and organic media. J Mol Catal B Enzym 133:533–542
Fernandez-Lafuente R (2009) Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb Technol 45:405–418
Padma VI, Laxmi A (2008) Enzyme stability and stabilization-Aqueous and non-aqueous environment. Process Biochem 43:1019–1032
Lian X, Fang Y, Joseph E, Wang Q, Li JL, Banerjee S, Lollar C, Wang X, Zhou HC (2017) Enzyme-MOF (metal–organic framework) composites. Chem Soc Rev 46:3386–3401
Cantone S, Ferrario V, Corici L, Ebert C, Fattor D, Spizzoa P, Gardossi L (2013) Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem Soc Rev 42:6262–6276
Pahujani S, Kanwar S, Chauhan G, Gupta R (2008) Glutaraldehyde activation of polymer Nylon-6 for lipase immobilization: Enzyme characteristics and stability. Bioresour Technol 99(7):2566–2570
Jiang DS, Long SY, Huang J, Xiao HY, Zhou JY (2005) Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem Eng J 25(1):15–23
Guzik U, Hupert-Kocurek K, Wojcieszyńska D (2014) Immobilization as a strategy for improving enzyme properties-application to oxidoreductases. Molecules 19:8995–9018
Balcão VM, Vila MM (2015) Structural and functional stabilization of protein entities: state-of-the-art. Adv Drug Deliv Rev 93:25–41
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microbial Technol 40:1451–1463
DiCosimo R, McAuliffe J, Pouloseb AJ, Bohlmann G (2013) Industrial use of immobilized enzymes. Chem Soc Rev 42:6437–6474
Liese A, Hilterhaus L (2013) Evaluation of immobilized enzymes for industrial applications. Chem Soc Rev 42:6236–6249
Rueda N, Santos CS, Rodriguez MD, Albuquerque TL, Barbosa O, Torres R, Ortiz C, Fernandez-Lafuente R (2016) Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J Mol Catal B Enzym 128:10–18
Rodrigues RC, Ortiz C, Berenguer-Murcia A, Torresd R, Fernandez-Lafuente R (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42:6290–6307
Francesco S (2013) Conformational changes of enzymes upon immobilization. Chem Soc Rev 42:6250–6261
Liu X, Lei L, Li YF, Zhu H, Cui YJ, Hu HY (2011) Preparation of carriers based on magnetic nanoparticles grafted polymer and immobilization for lipase. Biochem Eng J 56(3):142–149
Cipolatti EP, Valerio A, Henriques RO, Moritz DE, Ninow JL, Freire DMG, Manoel EA, Fernandez-Lafuente R, Oliveira D (2016) Nanomaterials for biocatalyst immobilization-state of the art and future trends. RSC Adv 6:104675–104692
Vaghari H, Jafarizadeh-Malmiri H, Mohammadlou M, Berenjian A, Anarjan N, Jafari N, Nasiri S (2016) Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol Lett 38:223–233
Hwang ET, Gu MB (2013) Enzyme stabilization by nano/microsized hybrid materials. Eng Life Sci 13(1):49–61
Ding Y, Hu Y, Zhang LY, Chen Y, Jiang XQ (2006) Synthesis and magnetic properties of biocompatible hybrid hollow spheres. Biomacromolecules 7(6):1766–1772
Guo Z, Bai S, Sun Y (2003) Preparation and characterization of immobilized lipase on magnetic hydrophobic microspheres. Enzyme Microb Tech 32(7):776–782
Liao MY, Huang CC, Chang MC, Lin SF, Liu TY, Su CH, Yeh CS, Lin HP (2011) Synthesis of magnetic hollow nanotubes based on the kirkendall effect for MR contrast agent and colorimetric hydrogen peroxide sensor. J Mater Chem 21:7974–7981
Li ZQ, Xie Y, Xiong YJ, Zhang R (2003) A novel non-template solution approach to fabricate ZnO hollow spheres with a coordination polymer as a reactant. New J Chem 27(10):1518–1521
Vasquez Y, Sra AK, Schaak RE (2005) One-Pot Synthesis of Hollow Superparamagnetic CoPt Nanospheres. J Am Chem Soc 127(36):12504–12505
Zhang Y, Li G, Zhang L (2004) Synthesis of indium hollow spheres and nanotubes by a simple template-free solvothermal process. Inorg Chem Commun 7(3):344–346
Gou L, Liang F, Wen XG, Yang SH, He L, Zheng WZ, Chen CP, Zhong QP (2002) Opal circuits of light—planarized microphotonic crystal chips. Adv Funct Mater 17(6–7):425–431
Gulay B, Yagmur T, Arica MY (2007) Immobilization of β-galactosidase onto magnetic poly(GMA–MMA) beads for hydrolysis of lactose in bed reactor. Catal Commun 8(7):1094–1101
Bayramoglu G, Kiralp S, Yilmaz M, Toppare L, Arıca MY (2008) Covalent immobilization of chloroperoxidase onto magnetic beads: catalytic properties and stability. Biochem Eng J 38(2):180–188
Yang Y, Bai YX, Li YF, Lei L, Cui YJ, Xia CG (2008) Preparation and application of polymer-grafted magnetic nanoparticles for lipase immobilization. J Magn Magn Mater 320(19):2350–2355
Rodrigues RC, Berenguer-Murcia A, Fernandez-Lafuente R (2011) Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv Synth Catal 353:2216–2238
Hernandez K, Fernandez-Lafuente R (2011) Control of protein immobilization: coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microbial Technol 48:107–122
Brady D, Jordaan J (2009) Advances in enzyme immobilization. Biotechnol Lett 31:1639–1650
Liu X, Li YF, Zhu WW, Fu PF (2013) Building on size-controllable hollow nanospheres with superparamagnetism derived from solid Fe3O4 nanospheres: preparation, characterization and application for lipase immobilization. Cryst Eng Commun 15:4937–4947
Xu FJ, Cai QJ, Li YL, Kang ET, Neoh KG (2005) Covalent immobilization of glucose oxidase on well-defined poly(glycidyl methacrylate)—Si(111) hybrids from surface-initiated atom-transfer radical polymerization. Biomacromolecules 6(2):1012–1020
Milosavić N, Prodanović R, Jovanović S, Vujčić Z (2007) Immobilization of glucoamylase via its carbohydrate moiety on macroporous poly(GMA-co-EGDMA). Enzyme Microb Tech 40(5):1422–1426
Teke AB, Baysal SH (2007) Immobilization of urease using glycidyl methacrylate grafted nylon-6-membranes. Process Biochem 42:439–443
Dradford MM (1976) A rapid and sensitive method for the quantitation of microgram. Anal Biochem 72:248–254
Stephanie P, Susan M, De P, Janos V, Nichol DS, Marcus T (2003) Poly(l-lysine)-graft-poly(ethylene glycol) assembled monolayers on niobium oxide surfaces: a quantitative study of the influence of polymer interfacial architecture on resistance to protein adsorption by ToF-SIMS and in Situ OWLS. Langmuir 19(22):9216–9225
Qu XF, Yao QZ, Zhou GT, Fu SQ, Huang JL (2010) Formation of hollow magnetite microspheres and their evolution into durian-like architectures. J Phys Chem C 114:8734–8740
Mooney KE, Nelson JA, Wagner M (2004) Superparamagnetic cobalt ferrite nanocrystals synthesized by alkalide reduction. Chem Mater 16(16):3155–3161
Yang XY, Chen L, Han B, Yang XL, Duan HQ (2010) Preparation of magnetite and tumor dual-targeting hollow polymer microspheres with pH-sensitivity for anticancer drug-carriers. Polymer 51(12):2533–2539
Wang XC, Liu J, Feng XF, Guo MJ, Sun DL (2008) Fabrication of hollow Fe3O4-polyaniline spheres with sulfonated polystyrene templates. Mater Chem Phys 112(2):319–321
Lei L, Bai YX, Li YF, Yi LX, Yang Y, Xia CG (2009) Study on immobilization of lipase onto magnetic microspheres with epoxy groups. J Magn Magn Mater 321(4):252–258
Liu X, Chen X, Li YF, Cui YJ, Zhu H, Zhu WW (2012) Preparation of superparamagnetic sodium alginate nanoparticles for covalent immobilization of Candida rugosa lipase. J Nanopart Res 14:763
Sheldon RA, Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42:6223–6235
Demirciogi LH, Beyenal H, Tanyolace A, Hasirci N (1994) Immobilization of urease and estimation of effective diffusion coefficients of urea in HEMA and VP copolymer matrices. Polym Int 35:321–327
Garcia-Galan C, Berenguer-Murcia A, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904
Arica MY, Hasirci V, Alaeddinoglu NG (1995) Covalent immobilization of α-amylase onto pHEMA microspheres: preparation and application to fixed bed reactor. Biomaterials 16(10):761–768
Yang Y, Bai YX, Li YF, Lei L, Cui YJ, Xia CG (2008) Characterization of Candida rugosa lipase immobilized onto magnetic microspheres with hydrophilicity. Process Biochem 43(11):1179–1185
Acknowledgements
This paper is dedicated to the memory of Prof. Yanfeng Li. The author thanks the financial supports from the National Natural Science Foundation of Ningxia (NZ14104), Key Scientific Research Project Foundation of North Minzu University (2015KJ25) and Foundation of Key Laboratory of Powder Materials and Special Ceramics (1405).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X. Preparation of porous hollow Fe3O4/P(GMA–DVB–St) microspheres and application for lipase immobilization. Bioprocess Biosyst Eng 41, 771–779 (2018). https://doi.org/10.1007/s00449-018-1910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1910-7