Abstract
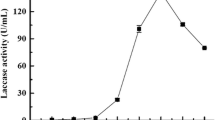
A strain of Ganoderma lucidum was separated and identified according to its morphological characteristics and phylogenetic data. The fungus is a laccase producer and it can secrete laccase using the municipal food waste (FW) as carbon and nitrogen supplement. After the statistic optimization, a laccase activity of 42,000 ± 600 U/l was obtained at 500 ml flask level and the activity is 12,000 U/l higher than that obtained by fermenting glucose and peptone, indicating that the use of FW to produce laccase not only reduces production cost, but also improves laccase activity. In 15 l bioreactor, FW is also suitable for laccase production and the maximum laccase activity reached 54,000 U/l. Moreover, some details of laccase overproduction using FW were investigated. The G. lucidum consumes FW by secreting a series of hydrolases and proteases and the improvement of laccase activity is because FW induces over-expression of three isoenzymes by polyacrylamide gel electrophoresis analysis.







Similar content being viewed by others
References
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 40:19–26
Baldrian P (2006) Fungal laccases occurrences and properties. FEMS Microbiol Rev 30:215–242
Couto SR, Toca-Herrera JL (2007) Laccase production at reactor scale by filamentous fungi. Biotechnol Adv 25:558–569
Stanescu MD, Gavrilas S, Ludwig R, Haltrich D, Lozinsky VI (2012) Preparation of immobilized Trametes pubescens laccase on a cryogel-type polymeric carrier and application of the biocatalyst to apple juice phenolic compounds oxidation. Eur Food Res Technol 234:655–662
Susana RC, Jose LTH (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Gao H, Chu X, Wang Y, Zhou F, Zhao K, Mu Z, Liu Q (2013) Media optimization for laccase production by Trichoderma harzianum ZF-2 using response surface methodology. J Microbiol Biotechnol 23:1757–1764
Liu LH, Lin ZW, Zheng T, Lin L, Zheng CQ, Lin ZX, Wang SH, Wang ZH (2009) Fermentation optimization and characterization of the laccase from Pleurotus ostreatus strain 10969. Enzyme Microb Technol 44:426–433
Dhakar K, Jain R, Tamta S, Pandey A (2014) Prolonged laccase production by a cold and pH tolerant strain of Penicillium pinophilum (MCC 1049) isolated from a low temperature environment. Enzyme Res 2014:e12078
Kiiskinen LL, Ratto M, Kruus K (2004) Screening for novel laccase-producing microbes. J Appl Microbiol 97:640–646
Sun J, Peng RH (2012) Secretory expression and characterization of a soluble laccase from the Ganoderma lucidum strain 7071-9 in Pichia pastoris. Mol Biol Rep 39:3807–3814
Theerachat M, Emond S, Cambon E, Bordes F, Marty A, Nicaud JM, Chulalaksananukul W, Guieysse D, Remaud-Simeon M, More S (2012) Engineering and production of laccase from Trametes versicolor in the yeast Yarrowia lipolytica. Bioresour Technol 125:267–274
Mishra A, Kumar S (2007) Cyanobacterial biomass as N-supplement to agro-waste for hyper-production of laccase from Pleurotus ostreatus in solid state fermentation. Process Biochem 42:681–685
Rosales E, Couto SR, Sanromán M (2005) Reutilisation of food processing wastes for production of relevant metabolites: application to laccase production by Trametes hirsute. J Food Eng 66:419–423
Songulashvili G, Elisashvili V, Wasser SP, Nevo E, Hadar Y (2007) Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzyme Microb Technol 41:57–61
Hu XJ, Zhang M, Yu JF, Zhang GR (2012) Food waste management in China: status, problem and solutions. Acta Ecologica Sinica 32:4575–4584
Yan SB, Li J, Chen XS, Wu JY, Wang PC, Ye JF, Yao JM (2011) Enzymatical hydrolysis of food waste and ethanol production from the hydrolysate. Renew Energy 36:1259–1265
Wataru N, Hazel BG, Hideki S, Yoichi N, Mitsumasa O (2004) Improvement of biological solubilization and mineralization process for food waste. J Water Environ Technol 2:57–64
Kiran EU, Trzcinski AP, Ng WJ, Liu Y (2014) Bioconversion of food waste to energy: a review. Fuel 134:389–399
Jiang Y, Yao YJ (2005) ITS sequence analysis and ascomatal development of Pseudogymnoascus roseus. Mycotaxon 94:55–73
Hai BZ, Liu L, Qin G, Peng YW, Li P, Yang QX, Wang HL (2014) Simulation of wastewater treatment by aerobic granules in a sequencing batch reactor based on cellular automata. Bioprocess Biosyst Eng. doi:10.1007/s00449-014-1181-x
APHA (1998) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–23
Chinese National Standard GB/T 5009.10 (2003) Determination of crude fiber in vegetable foods. Standards Press of China, Beijing
Chinese National Standard GB/T 5009.6 (2003) Determination of fat in foods. Standards Press of China, Beijing
Chinese National Standard GB/T 28715 (2012) Determination of acidic and neutral protease activity in feed additives—spectrophotometric method. Standards Press of China, Beijing
Chul SS, Hyung JK, Moon JK, Jae YJ (1998) Morphological change and enhanced pigment production of monascus when cocultured with Saccharomyces cerevisiae or Aspergillus oryzae. Biotechnol Bioeng 5:576–581
Reczey K, Szengyel Z, Eklund R, Zacchi G (1996) Cellulase production by T. reesei. Bioresour Technol 57:23–30
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates, an expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Wei JC (1979) The identification manual of fungi. Shanghai Scientific & Technical Publishers, Shanghai
Murugesan K, Nam IH, Kim YM, Chang YS (2007) Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzyme Microb Technol 40:1662–1672
Manavalan T, Manavalan A, Thangavelu KP, Heese K (2013) Characterization of optimized production, purification and application of laccase from Ganoderma lucidum. Biochem Eng J 70:106–114
Brückner R, Titgemeyer F (2002) Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett 209:141–148
Wang HL, Li P, Liu YF, Ren ZF, Wang G (2012) Overproduction of a potential red pigment by a specific self-immobilization biomembrane-surface liquid culture of Penicillium novae-zeelandiae. Bioprocess Biosyst Eng 35:1407–1416
Alberti A, Zielinski AAF, Zardo DM, Demiate IM, Nogueira A, Mafra LI (2014) Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem 49:151–158
Wang HL, Ren ZF, Li P, Gu YC, Liu GS, Yao JM (2011) Improvement of the production of a red pigment in Penicillium sp. HSD07B synthesized during co-culture with Candida tropicalis. Bioresour Technol 102:6082–6087
Patel MJ, Ng JHY, Hawkins WE, Pitts KF, Chakrabarti-Bell S (2012) Effects of fungal α-amylase on chemically leavened wheat flour doughs. J Cereal Sci 56:644–651
Okeke BC, Obi SKC (1995) Saccharification of agro-waste materials by fungal cellulases and hemicellulases. Bioresour Technol 51:23–27
Ko EM, Leem YE, Choi H (2001) Purification and characterization of laccase isozymes from the white-rot basidiomycete. Appl Microbiol Biotechnol 57:98–102
Wang HL, Tang CZ, Yu GL, Li P (2013) A novel membrane-surface liquid co-culture to improve the production of laccase from Ganoderma lucidum. Biochem Eng J 80:27–36
Acknowledgments
This work was supported by National Science Foundation of China (No. 51008119), and the fund for key subject of ecology, Henan Normal University, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hailei, W., Ping, L., Yuhua, Y. et al. Overproduction of laccase from a newly isolated Ganoderma lucidum using the municipal food waste as main carbon and nitrogen supplement. Bioprocess Biosyst Eng 38, 957–966 (2015). https://doi.org/10.1007/s00449-014-1341-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1341-z