Abstract
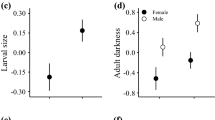
Clinal variation in body size and related life history traits is common and has stimulated the postulation of several eco-geographical rules. Whereas some clinal patterns are clearly adaptive, the causes of others remain obscure. We investigated intra-specific body size, development time and female fecundity (egg size and number) clines across 13 European populations of the dung fly Sepsis fulgens spanning 20° latitude from southern Italy to Estonia in a genetic common garden approach. Despite very short generation times (ca. 2 weeks at 24 °C), we found a converse Bergmann cline (smaller size at higher latitudes). As development time did not change with latitude (flat cline), integral growth rate thus likely declines towards the pole. At the same time, early fecundity, but not egg size, increased with latitude. Rather than being mediated by seasonal time constraints, the body size reduction in the northernmost flies from Estonia could suggest that these are marginal, edge populations, as when omitting them the body size cline became flat as well. Most of the other sepsid species investigated to date also show flat body size clines, a pattern that strikingly differs from Drosophila. We conclude that S. fulgens life history traits appear to be shaped by similar environmental pressures and selective mechanisms across Europe, be they adaptive or not. This reiterates the suggestion that body size clines can result as a secondary consequence of selection pressures shaping an entire life history syndrome, rendering them inconsistent and unpredictable in general.




Similar content being viewed by others
References
Ang YC, Puniamoorthy J, Pont AC, Bartak M, Blanckenhorn WU, Eberhard WG, Puniamoorthy N, Silva V, Munari L, Meier R (2013) Sepsidnet: a plea for digital reference collections and other science-based digitization initiatives in taxonomy. Syst Entomol 38:637–644
Armbruster P, Bradshaw WE, Ruegg K, Holzapfel CM (2001) Geographic variation and the evolution of reproductive allocation in the pitcher-plant mosquito, Wyeomyia smithii. Evolution 55:439–444
Arthur AL, Weeks AR, Sgro CM (2008) Investigating latitudinal clines for life history and stress resistance traits in Drosophila simulans from eastern Australia. J Evol Biol 21:1470–1479
Atkinson D (1994) Temperature and organism size a biological law for ectotherms? Adv Ecol Res 25:1–58
Atkinson D, Sibly RM (1997) Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol Evol 12:235–239
Azevedo RBR, French V, Partridge L (1996) Thermal evolution of egg size in Drosophila melanogaster. Evolution 50:2338–2345
Bauerfeind SS, Schäfer MA, Berger D, Blanckenhorn WU, Fox CW (2018) Replicated latitudinal clines in reproductive traits of European and North American yellow dung flies. Oikos (in press)
Berger D, Walters RJ, Gotthard K (2008) What limits insect fecundity? Body size- and temperature-dependent egg maturation and oviposition in a butterfly. Funct Ecol 22:523–529
Berger D, Postma E, Blanckenhorn WU, Walters RJ (2013) Quantitative genetic divergence and standing genetic (co)variance in thermal reaction norms along latitude. Evolution 67:2385–2399
Bergmann C (1847) Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Stud 3:595–708
Blackburn TM, Gaston KJ, Loder N (1999) Geographic gradients in body size: a clarification of Bergmann’s rule. Divers Distrib 5:165–174
Blanckenhorn WU (1999) Different growth responses to food shortage and temperature in three insect species with similar life histories. Evol Ecol 13:395–409
Blanckenhorn WU (2000) Temperature effects on egg size and their fitness consequences in the yellow dung fly. Evol Ecol 14:627–643
Blanckenhorn WU (2007) Case studies of the differential equilibrium hypothesis of sexual size dimorphism in dung flies. In: Fairbairn DJ, Blanckenhorn WU (eds) Sex, size and gender roles. Evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford, pp 106–114
Blanckenhorn WU, Demont M (2004) Bergmann and converse Bergmann latitudinal clines in Arthropods: two ends of a continuum? Integr Comp Biol 44:413–424
Blanckenhorn WU, Fairbairn DJ (1995) Life history adaptation along a latitudinal cline in water striders. J Evol Biol 8:21–41
Blanckenhorn WU, Heyland A (2004) The quantitative genetics of two life history trade-offs in the yellow dung fly in abundant and limited food environments. Evol Ecol 18:385–402
Blanckenhorn WU, Bauerfeind SS, Berger D, Davidowitz G, Fox CW, Guillaume F, Nakamura S, Nishimura K, Sasaki H, Stillwell RC, Tachi T, Schäfer MA (2018) Season length—not temperature—drives life-history clines on three continents in a widespread dungfly. Ecography. https://doi.org/10.1111/ecog.03752
Busso JP, Blanckenhorn WU (2018) Climatic factors shaping plastic trade-offs in the polyphenic black scavenger fly Sepsis thoracica (Diptera: Sepsidae). J Biogeo 45:593–603. https://doi.org/10.1111/jbi.13140
Calboli FCF, Gilchrist GW, Partrige L (2003) Different cell size and cell number contribution in two newly established and one ancient body size cline of Drosophila subobscura. Evolution 57:566–573
Chown SL, Gaston KJ (1999) Exploring links between physiology and ecology at macro-scales: the role of respiratory metabolism in insects. Biol Rev 74:87–120
Chown SL, Gaston KJ (2010) Body size variation in insects: a macroecological perspective. Biol Rev 85:139–169
Dmitriew C, Blanckenhorn WU (2012) The role of sexual selection and conflict in mediating among-population variation in mating strategies and sexually dimorphic traits in the black scavenger fly Sepsis punctum. PlosOne 7:e49511
Esperk T, Kjaersgaard A, Walters RJ, Berger D, Blanckenhorn WU (2016) Plastic and evolutionary responses to heat stress in a temperate dung fly: negative correlation between basal and induced heat tolerance? J Evol Biol 29:900–915
Fabian DK, Lack JB, Mathur V, Schloetterer C, Schmidt PS, Pool JE, Flatt T (2015) Spatially varying selection shapes life history clines among populations of Drosophila melanogaster from sub-Saharan Africa. J Evol Biol 28:826–840
Fischer K, Brakefield PM, Zwaan BJ (2003) Plasticity in butterfly egg size: why larger offspring at lower temperatures? Ecology 84:3138–3147
Flatt T (2016) Genomics of clinal variation in Drosophila: disentangling the interactions of selection and demography. Mol Ecol 25:1023–1026
Fox CW, Czesak ME (2000) Evolutionary ecology of progeny size in arthropods. Ann Rev Entomol 45:341–369
Garrad R, Booth DT, Furlong MJ (2016) The effect of rearing temperature on development, body size, energetics and fecundity of the diamondback moth. Bull Entomol Res 106:175–181
Hallas R, Schiffer M, Hoffmann AA (2002) Clinal variation in drosophila serrata for stress resistance and body size. Gen Res Camb 79:141–148
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hirst AG, Horne CR, Atkinson D (2015) Equal temperature-size responses of the sexes are widespread within arthropod species. Proc R Soc B 282:20152475. https://doi.org/10.1098/rspb.2015.2475
Honek A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492
Horne CR, Hirst AG, Atkinson D (2015) Temperature-size responses match latitudinal size clines in arthropods, revealing critical differences between aquatic and terrestrial species. Ecol Lett 18:327–335
Horne CR, Hirst AG, Atkinson D (2018) Insect temperature-body size trends common to laboratory, latitudinal and seasonal gradients are not found across altitudes. Funct Ecol. 32:948–957. https://doi.org/10.1111/1365-2435.13031
Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L (2000) Rapid evolution of a geographic cline in size in an introduced fly. Science 287:308–309
Imasheva AG, Bubli OA, Lazebny OE (1994) Variation in wing length in Eurasian natural poulations of Drosophila melanonogaster. Heredity 72:508–514
James FC (1970) Geographic size variation in birds and its relationship to climate. Ecology 51:365–390
James AC, Azevedo RBR, Partridge L (1997) Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics 146:881–890
Karan D, Munjal AK, Gibert P, Moreteau B, Parkash R, David JR (1998) Latitudinal clines for morphometrical traits in Drosophila kikkawai: a study of natural populations from the Indian subcontinent. Genet Res Camb 71:31–38
Karan D, Dubey S, Moreteau B, Parkash R, David JR (2000) Geographical clines for quantitative traits in natural populations of a tropical drosophilid: Zaprionus indianus. Genetica 108:91–100
Kivelä SM, Välimäki P, Carrasco D, Mäenpää MI, Oksanen J (2011) Latitudinal insect body size clines revisited: a critical evaluation of the saw-tooth model. J Anim Ecol 80:1184–1195
Klepsatel P, Galikova M, Huber CD, Flatt T (2014) Similarities and differences in altitudinal versus latitudinal variation for morphological traits in Drosophila melanogaster. Evolution 68:1385–1398
Lardies MA, Arias MB, Bacigalupe LD (2010) Phenotypic covariance matrix in life-history traits along a latitudinal gradient: a study case in a geographically widespread crab on the coast of Chile. Mar Ecol Prog Ser 412:179–187
Masaki S (1967) Geographic variation and climatic adaptation in a field cricket. Evolution 21:725–741
Misof B et al (2014) Phylogenomics resolves the timing and pattern of insect evolution. Science 346:763–767
Mousseau TA (1997) Ectotherms follow the converse to Bergmann’s rule. Evolution 51:630–632
Nygren GH, Bergström A, Nylin S (2008) Latitudinal body size clines in the butterfly Polyommatus icarus are shaped by gene–environment interactions. J Insect Science 8:47
Nylin S, Gotthard K (1998) Plasticity in life history traits. Ann Rev Entomol 43:63–83
Partridge L, Coyne JA (1997) Bergmann’s rule in ectotherms: is it adaptive? Evolution 51:632–635
Partridge L, Barrie B, Fowler K, French V (1994) Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48:1269–1276
Pont AC, Meier R (2002) The sepsidae (Diptera) of Europe. Fauna Entomol Scand 37:1–221
Puniamoorthy N, Rifqi M, Tan D, Meier R (2009) From kissing to belly stridulation: comparative analysis reveals surprising diversity, rapid evolution, and much homoplasy in the mating behavior of 27 species of sepsid flies (Diptera: Sepsidae). J Evol Biol 22:2146–2156
Puniamoorthy N, Schäfer MA, Blanckenhorn WU (2012) Sexual selection accounts for geographic reversal of sexual size dimorphism in the dung fly, Sepsis punctum (Diptera: Sepsidae). Evolution 66:2117–2126
Puniamoorthy N, Schäfer MA, Römbke J, Meier R, Blanckenhorn WU (2014) Ivermectin sensitivity is an ancient trait affecting all ecdysozoa but shows phylogenetic clustering among sepsid flies. Evol Appl 7:548–554
Roff D (1980) Optimizing development time in a seasonal environment: the ‘ups and downs’ of clinal variation. Oecologia 45:202–208
Rohner PT, Bächli G, Pollini Paltrinieri L, Duelli P, Obrist MK, Jochmann R, Blanckenhorn WU (2015) Distribution, diversity gradients and Rapoport’s elevational rule in the black scavenger flies of the Swiss Alps (Diptera: Sepsidae). Insect Conserv Divers 4:367–376
Rohner PT, Blanckenhorn WU, Puniamoorthy N (2016) Sexual selection on male size drives the evolution of male-biased sexual size dimorphism via the prolongation of male development. Evolution 70:1189–1199
Rohner PT, Blanckenhorn WU, Schäfer MA (2017) Critical weight mediates sex-specific body size plasticity and sexual dimorphism in the yellow dung fly Scathophaga stercoraria (Diptera: Scatophagidae). Evol Dev 19:147–156
Rohner PT, Pitnick S, Blanckenhorn WU, Snook RR, Bächli G, Lüpold S (2018a) Global macroecology of size, sexual size dimorphism, dispersal and range size in fruit flies (Diptera: Drosophilidae). Ecography. https://doi.org/10.1111/ecog.03382
Rohner PT, Teder T, Esperk T, Lüpold S, Blanckenhorn WU (2018b) The evolution of male-biased sexual size dimorphism is associated with increased body size plasticity in males. Funct Ecol 32:581–591. https://doi.org/10.1111/1365-2435.13004
Schilthuizen M, Kellermann V (2014) Contemporary climate change and terrestrial invertebrates: evolutionary versus plastic changes. Evol Appl 7:56–67
Shelomi M (2012) Where are we now? Bergmann’s rule sensu lato in insects. Am Nat 180:511–519
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Sota T, Takami Y, Kubota K, Ujiie M, Ishikawa R (2000) Interspecific body size differentiation in species assemblages of the carabid subgenus Ohomopterus in Japan. Pop Ecol 42:279–291
Stevenson RD (1985) Body size and limits to the daily range of body temperature in terrestrial ectotherms. Am Nat 125:102–117
Tammaru T, Esperk T (2007) Growth allometry of immature insects: larvae do not grow exponentially. Funct Ecol 21:1099–1105
Teuschl Y, Blanckenhorn WU (2007) The reluctant fly: what makes Sepsis cynipsea females willing to copulate? Anim Behav 73:85–97
van der Have TM, de Jong G (1996) Adult size in ectotherms: temperature effects on growth and differentiation. J Theor Biol 18:329–340
Van Voorhies WA (1996) Bergmann size clines: a simple explanation for their occurrence in ectotherms. Evolution 50:1259–1264
Zwaan BJ, Azevedo RBR, James AC, Van’t Land J, Partridge L (2000) Cellular basis of wing size variation in Drosophila melanogaster: a comparison of latitudinal clines on two continents. Heredity 84:338–347
Acknowledgements
We thank Martin Schäfer, Juan Pablo Busso, Anders Kjaersgaard, Nalini Puniamoorthy, Toomas Esperk, and Toomas Tammaru for collecting flies, and the entire Wolfpack for various support. This study was supported by Grant No. SNF 31003A-143787 from the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
JR, WUB and PTR conceived and designed the study, and contributed all to the statistical analysis and the writing of the manuscript. JR and PTR performed the experiments.
Corresponding author
Additional information
Communicated by Sylvain Pincebourde.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roy, J., Blanckenhorn, W.U. & Rohner, P.T. Largely flat latitudinal life history clines in the dung fly Sepsis fulgens across Europe (Diptera: Sepsidae). Oecologia 187, 851–862 (2018). https://doi.org/10.1007/s00442-018-4166-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-018-4166-7