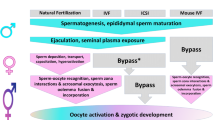
Abstract
Maternal mitochondrial inheritance is a fundamental paradigm within reproductive biology, yet the molecular mechanisms which underlie this process remain poorly understood. The ubiquitin proteasome system (UPS) and branches of the autophagic pathway have been implicated in taking part in the active degradation of sperm mitochondria post-fertilization. Despite this knowledge, there remains much unknown about this process, including the cofactors and substrates involved, as well as the implications of what occurs when these systems of degradation fail. Mitochondrial inheritance research has utilized a variety of animal models. However, one model that is of particular importance, especially when attempting to link mitochondrial inheritance research to humans, is the domestic pig. Pigs offer relatively easy collection of gametes which are similar to those of humans. Furthermore, pigs are physiologically and anatomically more similar to humans than the majority of other model systems available. Porcine in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), and novel cell-free systems are research tools which can be exploited to provide greater insight into the processes behind sperm mitochondrial degradation. In the future studies of mitochondrial inheritance, pigs will likely play a crucial role as an animal model system.


Similar content being viewed by others
References
Al Rawi S, Louvet-Vallee S, Djeddi A, Sachse M, Culetto E, Hajjar C, Boyd L, Legouis R, Galy V (2011) Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science 334:1144–1147
Albertini DF (2011) On the matter of Krogh’s principle. J Assist Reprod Genet 28:1–2
Antelman J, Manandhar G, Yi YJ, Li R, Whitworth KM, Sutovsky M, Agca C, Prather RS, Sutovsky P (2008) Expression of mitochondrial transcription factor a (TFAM) during porcine gametogenesis and preimplantation embryo development. J Cell Physiol 217:529–543
Archibald AL, Bolund L, Churcher C, Fredholm M, Groenen MA, Harlizius B, Lee KT, Milan D, Rogers J, Rothschild MF et al (2010) Pig genome sequence--analysis and publication strategy. BMC Genomics 11:438
Aw WC, Garvin MR, Ballard JWO (2019) Exogenous factors may differentially influence the selective costs of mtDNA mutations. Adv Anat Embryol Cell Biol
Bianchi E, Doe B, Goulding D, Wright GJ (2014) Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 508:483–487
Bianchi E, Wright GJ (2015) Cross-species fertilization: the hamster egg receptor, Juno, binds the human sperm ligand, Izumo1. Philos Trans R Soc Lond Ser B Biol Sci 370:20140101
Braude P, Bolton V, Moore S (1988) Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 332:459–461
Cummins JM, Woodall PF (1985) On mammalian sperm dimensions. J Reprod Fertil 75:153–175
Day BN (2000) Reproductive biotechnologies: current status in porcine reproduction. Anim Reprod Sci 60-61:161–172
Flach G, Johnson MH, Braude PR, Taylor RA, Bolton VN (1982) The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J 1:681–686
Galen (1586). Galeni Librorum Quarta Classis Apud luntas, Venetijs [Venice]
Giuffra E, Kijas JM, Amarger V, Carlborg O, Jeon JT, Andersson L (2000) The origin of the domestic pig: independent domestication and subsequent introgression. Genetics 154:1785–1791
Glotzer M, Murray AW, Kirschner MW (1991) Cyclin is degraded by the ubiquitin pathway. Nature 349:132–138
Hao Y, Mathialagan N, Walters E, Mao J, Lai L, Becker D, Li W, Critser J, Prather RS (2006) Osteopontin reduces polyspermy during in vitro fertilization of porcine oocytes. Biol Reprod 75:726–733
Herbert M, Turnbull D (2018) Progress in mitochondrial replacement therapies. Nat Rev Mol Cell Biol 19:71–72
Jansen RP (2000) Germline passage of mitochondria: quantitative considerations and possible embryological sequelae. Hum Reprod 15(Suppl 2):112–128
Jensen TW, Mazur MJ, Pettigew JE, Perez-Mendoza VG, Zachary J, Schook LB (2010) A cloned pig model for examining atherosclerosis induced by high fat, high cholesterol diets. Anim Biotechnol 21:179–187
Jonas E, de Koning DJ (2015) Genomic selection needs to be carefully assessed to meet specific requirements in livestock breeding programs. Front Genet 6:49
Kaneda H, Hayashi J, Takahama S, Taya C, Lindahl KF, Yonekawa H (1995) Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proc Natl Acad Sci U S A 92:4542–4546
Katayama M, Rieke A, Cantley T, Murphy C, Dowell L, Sutovsky P, Day BN (2007) Improved fertilization and embryo development resulting in birth of live piglets after intracytoplasmic sperm injection and in vitro culture in a cysteine-supplemented medium. Theriogenology 67:835–847
Kerns K, Zigo M, Drobnis EZ, Sutovsky M, Sutovsky P (2018) Zinc ion flux during mammalian sperm capacitation. Nat Commun 9:2061
Kong BY, Duncan FE, Que EL, Kim AM, O'Halloran TV, Woodruff TK (2014) Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol Hum Reprod 20:1077–1089
Kramer P, Bressan P (2018) Our (mother’s) mitochondria and our mind. Perspect Psychol Sci 13:88–100
Kramer P, Bressan P (2019) Mitochondria inspire a lifestyle. Adv Anat Embryol Cell Biol 231
Kuzmuk KN, Shook LB (2011) Pigs as a model for biomedical sciences. The Genetics of the Pig:426–444
Lee CN, Handrow RR, Lenz RW, Ax RL (1985) Interactions of seminal plasma and glycosaminoglycans on acrosome reactions in bovine spermatozoa in vitro. Gamete Res 12:345–355
Losano JDA, Padin JF, Mendez-Lopez I, Angrimani DSR, Garcia AG, Barnabe VH, Nichi M (2017) The stimulated glycolytic pathway is able to maintain ATP levels and kinetic patterns of bovine epididymal sperm subjected to mitochondrial uncoupling. Oxidative Med Cell Longev 2017:1682393
Luo S, Valencia CA, Zhang J, Lee NC, Slone J, Gui B, Wang X, Li Z, Dell S, Brown J et al (2018) Biparental inheritance of mitochondrial DNA in humans. Proc Natl Acad Sci U S A 115:13039–13044
Luo SM, Ge ZJ, Wang ZW, Jiang ZZ, Wang ZB, Ouyang YC, Hou Y, Schatten H, Sun QY (2013) Unique insights into maternal mitochondrial inheritance in mice. Proc Natl Acad Sci U S A 110:13038–13043
Maddox-Hyttel P, Dinnyes A, Laurincik J, Rath D, Niemann H, Rosenkranz C, Wilmut I (2001) Gene expression during pre- and peri-implantation embryonic development in pigs. Reprod Suppl 58:175–189
Mao J, O’Gorman C, Sutovsky M, Zigo M, Wells KD, Sutovsky P (2018) Ubiquitin A-52 residue ribosomal protein fusion product 1 (Uba52) is essential for preimplantation embryo development. Biol Open 7
Merlet J, Rubio-Pena K, Al Rawi S, and Galy V (2019) Autophagosomal sperm organelle clearance and mtDNA inheritance in C. elegans. Adv Anat Embryol Cell Biol, 1–24
Mio Y, Iwata K, Yumoto K, Kai Y, Sargant HC, Mizoguchi C, Ueda M, Tsuchie Y, Imajo A, Iba Y et al (2012) Possible mechanism of polyspermy block in human oocytes observed by time-lapse cinematography. J Assist Reprod Genet 29:951–956
Mtango NR, Sutovsky M, Susor A, Zhong Z, Latham KE, Sutovsky P (2012) Essential role of maternal UCHL1 and UCHL3 in fertilization and preimplantation embryo development. J Cell Physiol 227:1592–1603
Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y (1985) Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem 260:9699–9705
Peng L, Wen M, Liu Q, Peng J, Tang S, Hong Y, Liu S, Xiao Y (2018) Persistence and transcription of paternal mtDNA dependent on the delivery strategy rather than mitochondria source in fish embryos. Cell Physiol Biochem 47:1898–1908
Politi Y, Gal L, Kalifa Y, Ravid L, Elazar Z, Arama E (2014) Paternal mitochondrial destruction after fertilization is mediated by a common endocytic and autophagic pathway in Drosophila. Dev Cell 29:305–320
Prather RS, Day BN (1998) Practical considerations for the in vitro production of pig embryos. Theriogenology 49:23–32
Pyle A, Hudson G, Wilson IJ, Coxhead J, Smertenko T, Herbert M, Santibanez-Koref M, Chinnery PF (2015) Extreme-depth re-sequencing of mitochondrial DNA finds no evidence of paternal transmission in humans. PLoS Genet 11:e1005040
Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP et al (2015) Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem 7:130–139
Renner S, Fehlings C, Herbach N, Hofmann A, von Waldthausen DC, Kessler B, Ulrichs K, Chodnevskaja I, Moskalenko V, Amselgruber W et al (2010) Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes 59:1228–1238
Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, Petroff E, Vermeer DW, Kabel AC, Yan Z et al (2008) Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest 118:1571–1577
Rojansky R, Cha MY, Chan DC (2016) Elimination of paternal mitochondria in mouse embryos occurs through autophagic degradation dependent on PARKIN and MUL1. Elife 5
Ross JW, Fernandez de Castro JP, Zhao J, Samuel M, Walters E, Rios C, Bray-Ward P, Jones BW, Marc RE, Wang W et al (2012) Generation of an inbred miniature pig model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 53:501–507
Sato M, Sato K (2011) Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science 334:1141–1144
Schook LB, Beattie C, Beever J, Donovan S, Jamison R, Zuckermann F, Niemi S, Rothschild M, Rutherford M, Smith D (2005a) Swine in biomedical research: creating the building blocks of animal models. Anim Biotechnol 16:183–190
Schook LB, Beever JE, Rogers J, Humphray S, Archibald A, Chardon P, Milan D, Rohrer G, Eversole K (2005b) Swine genome sequencing consortium (SGSC): a strategic roadmap for sequencing the pig genome. Comp Funct Genomics 6:251–255
Schwartz M, Vissing J (2002) Paternal inheritance of mitochondrial DNA. N Engl J Med 347:576–580
Sharpley MS, Marciniak C, Eckel-Mahan K, McManus M, Crimi M, Waymire K, Lin CS, Masubuchi S, Friend N, Koike M et al (2012) Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell 151:333–343
Shitara H, Kaneda H, Sato A, Inoue K, Ogura A, Yonekawa H, Hayashi JI (2000) Selective and continuous elimination of mitochondria microinjected into mouse eggs from spermatids, but not from liver cells, occurs throughout embryogenesis. Genetics 156:1277–1284
Shitara H, Kaneda H, Sato A, Iwasaki K, Hayashi J, Taya C, Yonekawa H (2001) Non-invasive visualization of sperm mitochondria behavior in transgenic mice with introduced green fluorescent protein (GFP). FEBS Lett 500:7–11
Song WH, Sutovsky P (2018) Porcine cell-free system to study mammalian sperm mitophagy. Methods Mol Biol
Song WH, Yi YJ, Sutovsky M, Meyers S, Sutovsky P (2016a) The ART and science of sperm mitophagy. Autophagy 12:2510–2511
Song WH, Yi YJ, Sutovsky M, Meyers S, Sutovsky P (2016b) Autophagy and ubiquitin-proteasome system contribute to sperm mitophagy after mammalian fertilization. Proc Natl Acad Sci U S A 113:E5261–E5270
Srirattana K, St John JC (2019) Transmission of dysfunctional mitochondrial DNA and its implications for mammalian reproduction. Adv Anat Embryol Cell Biol 231:75–104
St John JC, Srirattana K, Tsai TS, Sun X (2017) The mitochondrial genome: how it drives fertility. Reprod Fertil Dev 30:118–139
St John JC, Tsai TS (2018) The association of mitochondrial DNA haplotypes and phenotypic traits in pigs. BMC Genet 19:41
St John JC, Tsai TS, Cagnone GL (2016) Mitochondrial DNA supplementation as an enhancer of female reproductive capacity. Curr Opin Obstet Gynecol 28:211–216
Stricker-Krongrad A, Shoemake CR, Pereira ME, Gad SC, Brocksmith D, Bouchard GF (2016) Miniature swine breeds in toxicology and drug safety assessments: what to expect during clinical and pathology evaluations. Toxicol Pathol 44:421–427
Sutovsky P (2018) Pig overview (male reproduction). In: B Jegou B, skinner MK (eds), Encyclopedia of reproduction second edition vol. 1
Sutovsky P, Hewitson L, Simerly CR, Tengowski MW, Navara CS, Haavisto A, Schatten G (1996a) Intracytoplasmic sperm injection for Rhesus monkey fertilization results in unusual chromatin, cytoskeletal, and membrane events, but eventually leads to pronuclear development and sperm aster assembly. Hum Reprod 11:1703–1712
Sutovsky P, McCauley TC, Sutovsky M, Day BN (2003) Early degradation of paternal mitochondria in domestic pig (Sus scrofa) is prevented by selective proteasomal inhibitors lactacystin and MG132. Biol Reprod 68:1793–1800
Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G (1999) Ubiquitin tag for sperm mitochondria. Nature 402:371–372
Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G (2000) Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol Reprod 63:582–590
Sutovsky P, Navara CS, Schatten G (1996b) Fate of the sperm mitochondria, and the incorporation, conversion, and disassembly of the sperm tail structures during bovine fertilization. Biol Reprod 55:1195–1205
Sutovsky P, Schatten G (1997) Depletion of glutathione during bovine oocyte maturation reversibly blocks the decondensation of the male pronucleus and pronuclear apposition during fertilization. Biol Reprod 56:1503–1512
Sutovsky P, Song WH (2017) Post-fertilisation sperm mitophagy: the tale of mitochondrial Eve and Steve. Reprod Fertil Dev 30:56–63
Sutovsky P, Van Leyen K, McCauley T, Day BN, Sutovsky M (2004) Degradation of paternal mitochondria after fertilization: implications for heteroplasmy, assisted reproductive technologies and mtDNA inheritance. Reprod BioMed Online 8:24–33
Thompson WE, Ramalho-Santos J, Sutovsky P (2003) Ubiquitination of prohibitin in mammalian sperm mitochondria: possible roles in the regulation of mitochondrial inheritance and sperm quality control. Biol Reprod 69:254–260
Tsai T, St John JC (2016) The role of mitochondrial DNA copy number, variants, and haplotypes in farm animal developmental outcome. Domest Anim Endocrinol 56(Suppl):S133–S146
Tsang HG, Rashdan NA, Whitelaw CB, Corcoran BM, Summers KM, MacRae VE (2016) Large animal models of cardiovascular disease. Cell Biochem Funct 34:113–132
Uh K, Ryu J, Zhang L, Errington J, Machaty Z, Lee K (2019) Development of novel oocyte activation approaches using Zn(2+) chelators in pigs. Theriogenology 125:259–267
Varea-Sanchez M, Tourmente M, Bastir M, Roldan ER (2016) Unraveling the sperm bauplan: relationships between sperm head morphology and sperm function in rodents. Biol Reprod 95:25
Vissing J (2019) Paternal comeback in mitochondrial DNA inheritance. Proc Natl Acad Sci U S A 116:1475–1476
Wei Y, Chiang WC, Sumpter R Jr, Mishra P, Levine B (2017) Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 168(224–238):e210
Xia P (2013) Biology of polyspermy in IVF and its clinical indication. Curr Obstet Gynecol Reports 2:226–231
Yen, N.T., Lin, C.S., Ju, C.C., Wang, S.C., and Huang, M.C. (2007). Mitochondrial DNA polymorphism and determination of effects on reproductive trait in pigs. Reproduction in domestic animals = Zuchthygiene 42, 387-392
Yi YJ, Manandhar G, Sutovsky M, Li R, Jonakova V, Oko R, Park CS, Prather RS, Sutovsky P (2007) Ubiquitin C-terminal hydrolase-activity is involved in sperm acrosomal function and anti-polyspermy defense during porcine fertilization. Biol Reprod 77:780–793
Zhou Q, Li H, Nakagawa A, Lin JL, Lee ES, Harry BL, Skeen-Gaar RR, Suehiro Y, William D, Mitani S et al (2016) Mitochondrial endonuclease G mediates breakdown of paternal mitochondria upon fertilization. Science 353:394–399
Zhou Q, Li H, Xue D (2011) Elimination of paternal mitochondria through the lysosomal degradation pathway in C. elegans. Cell Res 21:1662–1669
Acknowledgements
We thank Professor Rodney Geisert (MU Animal Science) for the image of the porcine female reproductive tract incorporated in Figure 1, and Professor Kathy Timms (MU OBGYN & Women’s Health) for permission to use unpublished data from a joint research project (Figure 2B). We appreciate our colleagues and collaborators, past and present who have been supporting our research on sperm mitophagy. Porcine gametes for our work have been provided reliably by the NIH National Swine Resource and Research Center (NSRRC), University of Missouri.
Funding
Work in our laboratory, directly pertinent to this manuscript has been funded by Agriculture and Food Research Initiative Competitive Grant no. 2013-67015-20961. Other research in our laboratory relevant to this review has been funded by the National Institute of Food and Agriculture (NIFA), U.S. Department of Agriculture (USDA) grant number 2015-67015-23231, grant number 5 R01 HD084353-02 from NIH National Institute of Child and Human Development, and seed funding from the Food for the twenty-first Century Program of the University of Missouri.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Not applicable.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zuidema, D., Sutovsky, P. The domestic pig as a model for the study of mitochondrial inheritance. Cell Tissue Res 380, 263–271 (2020). https://doi.org/10.1007/s00441-019-03100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03100-z