Abstract
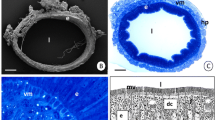
Defoliation caused by Anticarsia gemmatalis larvae affects the commercial production of the soybean. Although regulation of the digestion of soybean components has become part of the suggested strategy to overcome problems caused by Anticarsia larvae, few studies have focused on the morphological and cellular aspects of Anticarsia intestinal tissue. We have therefore further analyzed the morphology and ultrastructure of the midgut of 5th instar larvae of A. gemmatalis. Dissected midgut was subjected to chemical or cryo-fixation and then to several descriptive and analytical techniques associated with both light and electron microscopy in order to correlate anatomical and physiological aspects of this organ. Histological analysis revealed typical anatomy composed of a cell layer limited by a peritrophic membrane. The identified lepidoptera-specific goblet cells were shown to contain several mitochondria inside microvilli of the goblet cell cavity and a vacuolar H+-ATPase possibly coupled to a K+-pumping system. Columnar cells were present and exhibited microvilli dispersed along the apical region that also presented secretory characteristics. We additionally found evidence for the secretion of polyphosphate (PolyP) into the midgut, a result corroborating previous reports suggesting an excretion route from the goblet cell cavity toward the luminal space. Thus, our results suggest that the Anticarsia midgut not only possesses several typical lepidopteran features but also presents some unique aspects such as the presence of a tubular network and PolyP-containing apocrine secretions, plus an apparent route for the release of cellular debris by the goblet cells.






Similar content being viewed by others
References
Anderson E, Harvey W (1966) Active transport by the Cecropia midgut. J Cell Biol 31:107–134
Azuma M, Harvey W, Wieczorek H (1995) Stoichiometry of K+/H+ antiport helps to explain extracellular pH 11 in a model epithelium. FEBS Lett 361:153–156
Baldwin K, Hakim R (1991) Growth and differentiation of the larval midgut epithelium during molting in the moth, Manduca sexta. Tissue Cell 23:411–422
Berner R, Rudin W, Hecker H (1983) Peritrophic membranes and protease activity in the midgut of the malaria mosquito, Anopheles stephensi (Liston) (Insecta: Diptera) under normal and experimental conditions. J Ultrastruct Res 83:195–204
Bignell DE, Oskarsson H, Anderson JM (1982) Formation of membrane-bounded secretory granules in the midgut epithelium of a termite, Cubitermes severus, and a possible intercellular route of discharge. Cell Tissue Res 222:187–200
Bird CH, Sutton VR, Sun J, Hirst CE, Novak A, Kumar S, Trapani JA, Bird PI (1998) Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme B-mediated apoptosis without perturbing the Fas cell death pathway. Mol Cell Biol 18:6387–6398
Church FC, Pratt CW, Treanor RE, Whinna HC (1988) Antithrombin action of phosvitin and other phosphate-containing polyanions is mediated by heparin cofactor II. FEBS Lett 237:26–30
Cioffi M (1979) The morphology and fine structure of the larval midgut of a moth (Manduca sexta) in relation to active ion transport. Tissue Cell 11:467–479
Cioffi M (1984) Comparative ultrastructure of arthropod transporting epithelia. Am Zool 24:139–156
Cioffi M, Harvey WR (1981) Comparison of potassium transport in three structurally distinct regions of the insect midgut. J Exp Biol 91:103–116
Dames P, Zimmermann B, Schmidt R, Rein J, Voss M, Schewe B, Walz B, Baumann O (2006) cAMP regulates plasma membrane vacuolar-type H+-ATPase assembly and activity in blowfly salivary glands. Proc Natl Acad Sci USA 103:3926–3931
Dow JAT(1986) Insect midgut function. In: Evans PD, Wigglesworth VB (eds) Advances in insect physiology, vol 19. Academic Press, London, pp 187-328
Dow J (1992) pH gradients in lepidopetran midgut. J Exp Biol 172:355–375
Dow J, Gupta B, Hall T, Harvey W (1984) X-ray microanalysis of elements in frozen-hydrated sections of an electrogenic K+ transport system: the posterior midgut of tobacco hornworm (Manduca sexta) in vivo and in vitro. J Membr Biol 77:223–241
Espinoza-Fuentes F, Terra WR (1987) Physiological adaptations for digesting bacteria. Water fluxes and distribution of digestive enzymes in Musca domestica larval midgut. Insect Biochem 17:809–817
Giordana B, Leonardi M, Casartelli M, Consonni P, Parenti P (1998) K+-neutral amino acid symport of Bombyx mori larval midgut: a system operative in extreme conditions. Am J Physiol 274:R1361-R1371
Gomes FM, Ramos IB, Motta LM, Miranda K, Santiago MF, de Souza W, Machado EA (2008) Polyphosphate polymers during early embryogenesis of Periplaneta americana. J Insect Physiol 54:1459–1466
Gomes FM, Oliveira DMP, Motta LS, Ramos IB, Miranda KM, Machado EA (2010) Inorganic polyphosphate inhibits an aspartic protease-like activity in the eggs of Rhodnius prolixus (Stahl) and impairs yolk mobilization in vitro. J Cell Physiol 222:606–611
Gomes FM, Carvalho DB, Peron AC, Saito K, Miranda K, Machado EA (2012) Inorganic polyphosphates are stored in spherites within the midgut of Anticarsia gemmatalis and play a role in copper detoxification. J Insect Physiol 58:211–219
Graf R, Novak F, Harvey W, Wieczorek H (1992) Cloning and sequencing of cDNA encoding the putative insect plasma membrane V-ATPase subunit A. FEBS Lett 300:119–122
Graf R, Harvey W, Wieczorek H (1994a) Cloning, sequencing and expression of cDNA encoding an insect V-ATPase subunit E. Biochim Biophys Acta 1190:193–196
Graf R, Lepier A, Harvey W, Wieczorek H (1994b) A novel 14-kDa V-ATPase subunit in the tobacco hornworm midgut. J Biol Chem 269:3767–3774
Grinstein S, Wieczorek H (1994) Cation antiports of animal plasma membranes. J Exp Biol 196:307–318
Grosse AS, Pressprich MF, Curley LB, Hamilton KL, Margolis B, Hildebrand JD, Gumucio DL (2011) Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development 138:4423–4432
Hanozet G, Giordana B, Sacchi V (1980) K+-dependent phenylalanine uptake in membrane vesicles isolated from the midgut of Philosamia cynthia larvae. Biochim Biophys Acta 596:481–486
Hardt M, Plattner H (2000) Sub-second quenched-flow/X-ray microanalysis shows rapid Ca2+ mobilization from cortical stores paralleled by Ca2+ influx during synchronous exocytosis in Paramecium cells. Eur J Cell Biol 79:642–652
Harvey W, Nedergaard S (1964) Sodium-independent active transport of potassium in the isolated midgut of the Cecropia silkworm. Proc Natl Acad Sci USA 51:757–765
Harvey W, Cioffi M, Wolfersberger M (1981) Portasomes as coupling factors in active ion transport and oxidative phosphorylation. Am Zool 21:775–791
Harvey W, Cioffi M, Dow J, Wolfersberger M (1983) Potassium ion transport ATPase in insect epithelia. J Exp Biol 106:91–117
Harvey WR, Boudko DY, Rheault MR, Okech BA (2009) NHEVNAT: an H+ V-ATPase electrically coupled to a Na+:nutrient amino acid transporter (NAT) forms an Na+/H+ exchanger (NHE). J Exp Biol 212:347–357
Harvey WR, Okech BA, Linser PJ, Becnel JJ, Ahearn GA, Sterling KM (2010) H+ V-ATPase-energized transporters in brush border membrane vesicles from whole larvae of Aedes aegypti. J Insect Physiol 56:1377–1389
Hennigan BB, Wolfersberger MG, Harvey WR (1993a) Neutral amino acid symport in larval Manduca sexta midgut brush-border membrane vesicles deduced from cation-dependent uptake of leucine, alanine, and phenylalanine. Biochim Biophys Acta 1148:216–222
Hennigan BB, Wolfersberger MG, Parthasarathy R, Harvey WR (1993b) Cation-dependent leucine, alanine, and phenylalanine uptake at pH 10 in brush-border membrane vesicles from larval Manduca sexta midgut. Biochim Biophys Acta 1148:209–215
Humbert W (1979) The midgut of Tomocerus minor Lubbock (insecta, collembola): ultrastructure, cytochemistry, ageing and renewal during a moulting cycle. Cell Tissue Res 196:39–57
Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW (1997) The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci USA 94:14683–14688
Kamada S, Funahashi Y, Tsujimoto Y (1997) Caspase-4 and caspase-5, members of the ICE/CED-3 family of cysteine proteases, are CrmA-inhibitable proteases. Cell Death Differ 4:473–478
Korayem AM, Fabbri M, Takahashi K, Scherfer C, Lindgren M, Schmidt O, Ueda R, Dushay MS, Theopold U (2004) A Drosophila salivary gland mucin is also expressed in immune tissues: evidence for a function in coagulation and the entrapment of bacteria. Insect Biochem Mol Biol 34:1297–1304
Lehane MJ (1976) Digestive enzyme secretion in Stomoxys calcitrans (Diptera: Muscidae). Cell Tissue Res 170:275–287
Levy S, Falleiros A, Gregório E, Arrebola N, Toledo L (2004) The larval midgut of Anticarsia gemmatalis (Hübner)(Lepidoptera: Noctuidae): light and electron microscopy studies of the epithelial cells. Braz J Biol 64:633–638
Levy S, Falleiros Â, Moscardi F, Gregório E (2007) Susceptibility/resistance of Anticarsia gemmatalis larvae to its nucleopolyhedrovirus (AgMNPV): structural study of the peritrophic membrane. J Invertebr Pathol 96:183–186
Liu N, Raja SM, Zazzeroni F, Metkar SS, Shah R, Zhang M, Wang Y, Bromme D, Russin WA, Lee JC, Peter ME, Froelich CJ, Franzoso G, Ashton-Rickardt PG (2003) NF-kappa B protects from the lysosomal pathway of cell death. EMBO J 22:5313–5322
Mathialagan N, Hansen TR (1996) Pepsin-inhibitory activity of the uterine serpins. Proc Natl Acad Sci USA 93:13653–13658
Matos T, Giugliano L, Ribeiro B, Báo S (1999) Structural and ultrastructural studies of Anticarsia gemmatalis midgut cells infected with the baculovirus A. gemmatalis nucleopolyhedrovirus. Int J Insect Morphol Embryol 28:195–201
Medema JP, Schuurhuis DH, Rea D, Van Tongeren J, De Jong J, Bres SA, Laban S, Toes REM, Toebes M, Schumacher TNM (2001) Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J Exp Med 194:657–668
Meleshkevitch EA, Robinson M, Popova LB, Miller MM, Harvey WR, Boudko DY (2009) Cloning and functional expression of the first eukaryotic Na+-tryptophan symporter, AgNAT6. J Exp Biol 212:1559–1567
Merzendorfer H, Huss M, Schmid R, Harvey WR, Wieczorek H (1999) A novel insect V-ATPase subunit M9.7 is glycosylated extensively. J Biol Chem 274:17372–17378
Merzendorfer H, Reineke S, Zhao X, Jacobmeier B, Harvey W, Wieczorek H (2000) The multigene family of the tobacco hornworm V-ATPase: novel subunits a, C, D, H, and putative isoforms1. Biochim Biophys Acta 1467:369–379
Moffatt M, Lehane M (1990) Trypsin is stored as an inactive zymogen in the midgut of Stomoxys calcitrans. Insect Biochem 20:719–723
Okech B, Boudko D, Linser P, Harvey W (2008) Cationic pathway of pH regulation in larvae of Anopheles gambiae. J Exp Biol 211:957–968
Pinheiro D, Quagio-Grassiotto I, Gregório E (2008) Morphological regional differences of epithelial cells along the midgut in Diatraea saccharalis Fabricius (Lepidoptera: Crambidae) larvae. Neotrop Entomol 37:413–419
Ramos IB, Miranda K, Pace DA, Verbist KC, Lin F-Y, Zhang Y, Oldfield E, Machado EA, de Souza W, Docampo R (2010) Calcium- and polyphosphate-containing acidic granules of sea urchin eggs are similar to acidocalcisomes, but are not the targets for NAADP. Biochem J 429:485–495
Rein J, Zimmermann B, Hille C, Lang I, Walz B, Baumann O (2006) Fluorescence measurements of serotonin-induced V-ATPase-dependent pH changes at the luminal surface in salivary glands of the blowfly Calliphora vicina. J Exp Biol 209:1716–1724
Reineke S, Wieczorek H, Merzendorfer H (2002) Expression of Manduca sexta V-ATPase genes mvB, mvG and mvd is regulated by ecdysteroids. J Exp Biol 205:1059–1067
Rheault M, Okech B, Keen S, Miller M, Meleshkevitch E, Linser P, Boudko D, Harvey W (2007) Molecular cloning, phylogeny and localization of AgNHA1: the first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. J Exp Biol 210:3848–3861
Ribalta T, McCutcheon IE, Aldape KD, Bruner JM, Fuller GN (2004) The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. Am J Surg Pathol 28:1532–1536
Rost-Roszkowska MM, Vilimova J, Sosinka A, Skudlik J, Franzetti E (2012) The role of autophagy in the midgut epithelium of Eubranchipus grubii (Crustacea, Branchiopoda, Anostraca). Arthropod Struct Dev 41:271–279
Ruiz FA, Lea CR, Oldfield E, Docampo R (2004) Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem 279:44250–44257
Santos CD, Ferreira C, Terra WR (1983) Consumption of food and spatial organization of digestion in the cassava hornworm, Erinnyis ello. J Insect Physiol 29:707–714
Santos CD, Ribeiro AF, Ferreira C, Terra WR (1984) The larval midgut of the cassava hornworm (Erinnyis ello). Cell Tissue Res 237:565–574
Santos CD, Ribeiro AF, Terra WR (1986) Differential centrifugation, calcium precipitation, and ultrasonic disruption of midgut cells of Erinnyis ello caterpillars. Purification of cell microvilli and inferences concerning secretory mechanisms. Can J Zool 64:490–500
Schumaker T, Cristofoletti P, Terra W (1993) Properties and compartmentalization of digestive carbohydrases and proteases in Scaptotrigona bipunctata (Apidae: Meliponinae) larvae. Apidologie 24:3–17
Schweikl H, Klein U, Schindlbeck M, Wieczorek H (1989) A vacuolar-type ATPase, partially purified from potassium transporting plasma membranes of tobacco hornworm midgut. J Biol Chem 264:11136–11142
Silverman G, Whisstock J, Askew D, Pak S, Luke C, Cataltepe S, Irving J, Bird P (2004) Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci 61:301–325
Sousa ME, Santos FA, Wanderley-Teixeira V, Teixeira AA, de Siqueira HA, Alves LC, Torres JB (2010) Histopathology and ultrastructure of midgut of Alabama argillacea (Hubner) (Lepidoptera: Noctuidae) fed Bt-cotton. J Insect Physiol 56:1913–1919
Suminami Y, Nagashima S, Vujanovic N, Hirabayashi K, Kato H, Whiteside T (2000) Inhibition of apoptosis in human tumour cells by the tumour-associated serpin, SCC antigen-1. Br J Cancer 82:981–989
Takeda A, Yamamoto T, Nakamura Y, Takahashi T, Hibino T (1995) Squamous cell carcinoma antigen is a potent inhibitor of cysteine proteinase cathepsin L. FEBS Lett 359:78–80
Terra WR, Ferreira C (1994) Insect digestive enzymes: properties, compartmentalization and function. Comp Biochem Physiol [B] 109:1–62
Terra W, Ferreira C, De Bianchi A (1979) Distribution of digestive enzymes among the endo- and ectoperitrophic spaces and midgut cells of Rhynchosciara and its physiological significance. J Insect Physiol 25:487–494
Tewari M, Telford WG, Miller RA, Dixit VM (1995) CrmA, a poxvirus-encoded serpin, inhibits cytotoxic T-lymphocyte-mediated apoptosis. J Biol Chem 270:22705–22708
Thunberg L, Bäckström G, Lindahl U (1982) Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res 100:393–410
Tijssen JPF, Tijssen JPF, Beekes HW, Beekes HW, Van Steveninck J, Van Steveninck J (1982) Localization of polyphosphates in Saccharomyces fragilis, as revealed by 4′,6-diamidino-2-phenylindole fluorescence. Biochim Biophys Acta 721:394–398
Vitavska O, Wieczorek H, Merzendorfer H (2003) A novel role for subunit C in mediating binding of the H+-V-ATPase to the actin cytoskeleton. J Biol Chem 278:18499–18505
Wallace W, Schaefer LH, Swedlow JR (2001) A workingperson’s guide to deconvolution in light microscopy. Biotechniques 31:1076–1097
Wang P, Granados R (1997) An intestinal mucin is the target substrate for a baculovirus enhancin. Proc Natl Acad Sci USA 94:6977–6982
Wieczorek H, Putzenlechner M, Zeiske W, Klein U (1991) A vacuolar-type proton pump energizes K+/H+ antiport in an animal plasma membrane. J Biol Chem 266:15340–15347
Wieczorek H, Brown D, Grinstein S, Ehrenfeld J, Harvey W (1999) Animal plasma membrane energization by proton-motive V-ATPases. Bioessays 21:637–648
Wolfersberger M, Harvey W, Cioffi M (1982) Transepithelial potassium transport in insect midgut by an electrogenic alkali metal ion pump. Curr Topics Membr Transp 16:109–133
Wood J, Farrand P, Harvey W (1969) Active transport of potassium by the cecropia midgut. VI. Microelectrode potential profile. J Exp Biol 50:169–178
Zimmermann B (1997) Serotonin-induced intercellular calcium waves in salivary glands of the blowfly Calliphora erythrocephala. J Physiol 500:17–28
Acknowledgements
We express our gratitude to Prof. Helmut Wieczorek and Dr. Markus Huss, University of Osnabrück for providing antibodies of the subunit e M. sexta and to Dr. Eduardo Fox and Prof. Wanderley de Souza for proofreading.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by grants from the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, INCT de Entomologia Molecular and INCT de Biologia Estrutural e Bioimagem), Coordenação de Aperfeiçoamento do Pessoal de Nível Superior (CAPES), Fundação Carlos Chagas Filho de Amparo à Pesquisa de Estado do Rio de Janeiro (FAPERJ) and CAPES-Petrobrás.
The funders of this work had no role in the study design, data collection and analysis, decision to publish, or preparation of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental figure 1
a Whole tissue immunohistochemistry with anti-pHH3 of the gut of Anticarsia larvae under ecdysis showing a banding pattern at discrete regions of the gut. The black bars above mark the regions stained by the antibodies. The graph below represents the relative intensity of the staining along the gut axial length. b TEM image showing the organization of cells at the basal lamina during the 5th instar. Inset position of the regenerative cells (asterisk) close to the basal lamina (bl, BL) and below a goblet cell (gc) cavity. c TEM image showing cells during ecdysis from the 4th to 5th instar. Note that they present a densely packed organization (gc goblet cell). Bar: B – 5 μm, B, inset – 15 μm, C – 5 μm. (JPEG 40 kb)
Supplemental figure 2
Typical electron probe X-ray microanalysis of the vesicles depicted in Fig. 2 prepared from high-pressure frozen tissues, freeze-substituted in the presence of KF as a calcium precipitating agent. (JPEG 5 kb)
(MP4 5596 kb)
Rights and permissions
About this article
Cite this article
Gomes, F.M., Carvalho, D.B., Machado, E.A. et al. Ultrastructural and functional analysis of secretory goblet cells in the midgut of the lepidopteran Anticarsia gemmatalis . Cell Tissue Res 352, 313–326 (2013). https://doi.org/10.1007/s00441-013-1563-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1563-4