Abstract
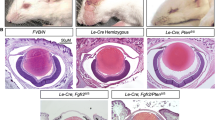
The Forkhead box E3 (FOXE3) gene encodes a transcription factor with a forkhead/winged helix domain that is critical for development of the lens and anterior segment of the eye. Monoallelic and biallelic deleterious sequence variants in FOXE3 cause aphakia, cataracts, sclerocornea and microphthalmia in humans. We used clustered regularly interspaced short palindromic repeats/Cas9 injections to target the foxe3 transcript in zebrafish in order to create an experimental model of loss of function for this gene. Larvae that were homozygous for an indel variant, c.296_300delTGCAG, predicting p.(Val99Alafs*2), demonstrated severe eye defects, including small or absent lenses and microphthalmia. The lenses of the homozygous foxe3 indel mutants showed more intense staining with zl-1 antibody compared to control lenses, consistent with increased lens fiber cell differentiation. Whole genome transcriptome analysis (RNA-Seq) on RNA isolated from wildtype larvae and larvae with eye defects that were putative homozygotes for the foxe3 indel variant found significant dysregulation of genes expressed in the lens and eye whose orthologues are associated with cataracts in human patients, including cryba2a, cryba1l1, mipa and hsf4. Comparative analysis of this RNA-seq data with iSyTE data identified several lens-enriched genes to be down-regulated in foxe3 indel mutants. We also noted upregulation of lgsn and crygmxl2 and downregulation of fmodb and cx43.4, genes that are expressed in the zebrafish lens, but that are not yet associated with an eye phenotype in humans. These findings demonstrate that this new zebrafish foxe3 mutant model is highly relevant to the study of the gene regulatory networks conserved in vertebrate lens and eye development.







Similar content being viewed by others
References
Amjadi S, Mai K, McCluskey P, Wakefield D (2013) The role of lumican in ocular disease. ISRN Ophthalmol 2013:632302
Anand D, Lachke SA (2017) Systems biology of lens development: a paradigm for disease gene discovery in the eye. Exp Eye Res 156:22–33
Anand D, Agrawal SA, Slavotinek A, Lachke SA (2018) Mutation Update of Transcription Factor Genes FOXE3, HSF4, MAF and PITX3 causing ocular defects. Hum Mutat 39:471–494
Behnam M, Imagawa E, Chaleshtori AR, Ronasian F, Salehi M, Miyake N, Matsumoto N (2016) A novel homozygous mutation in HSF4 causing autosomal recessive congenital cataract. J Hum Genet 61:177–179
Berthoud VM, Ngezahayo A (2017) Focus on lens connexins. BMC Cell Biol 18(Suppl 1):6
Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerbäck S, Carlsson P (2000) A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev 14:245–254
Blixt A, Landgren H, Johansson BR, Carlsson P (2007) Foxe3 is required for morphogenesis and differentiation of the anterior segment of the eye and is sensitive to Pax6 gene dosage. Dev Biol 302:218–229
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30:2114–2120
Brownell I, Dirksen M, Jamrich M (2000) Forkhead Foxe3 mapes to the dysgenetic lens locys and is critical in lens development and differentiation. Genesis 27:81–93
Chakravarti S, Paul J, Roberts L, Chervoneva I, Oldberg A, Birk DE (2003) Ocular and scleral alterations in gene-targeted lumican-fibromodulin double-null mice. Invest Ophthalmol Vis Sci 44:2422–2432
Chao R, Nevin L, Agarwal P, Riemer J, Bai X, Delaney A, Akana M, JimenezLopez N, Bardakjian T, Schneider A, Chassaing N, Schorderet DF, FitzPatrick D, Kwok PY, Ellgaard L, Gould DB, Zhang Y, Malicki J, Baier H, Slavotinek A (2010) A male with unilateral microphthalmia reveals a role for TMX3 in eye development. PLoS One 5:e10565
Chen S, Oldberg A, Chakravarti S, Birk DE (2010) Fibromodulin regulates collagen fibrillogenesis during peripheral corneal development. Dev Dyn 239:844–854
Chen J, Wang Q, Cabrera PE, Zhong Z, Sun W, Jiao X et al (2017) Molecular genetic analysis of Pakistani families with autosomal recessive congenital cataracts by homozygosity screening. Invest Ophthalmol Vis Sci 58:2207–2217
Chepelinsky AB (2009) Structural function of MIP/aquaporin 0 in the eye lens: genetic defects lead to congenital inherited cataracts. Handb Exp Pharmacol 190:265–297
Cvekl A, Zhang X (2017) Signaling and gene regulatory networks in mammalian lens development. Trends Genet 33:677–702
Cvekl A, McGreal R, Liu W (2015) Lens development and crystallin gene expression. Prog Mol Biol Transl Sci 134:129–167
Froger A, Clemens D, Kalman K, Németh-Cahalan KL, Schilling TF, Hall JE (2010) Two distinct aquaporin 0 s required for development and transparency of the zebrafish lens. Invest Ophthalmol Vis Sci 51:6582–6592
Fujimoto M, Izu H, Seki K, Fukuda K, Nishida T, Yamada S, Kato K, Yonemura S, Inouye S, Nakai A (2004) HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J 23:4297–4306
Gould DB, John SW (2002) Anterior segment dysgenesis and the developmental glaucomas are complex traits. Hum Mol Genet 11:1185–1193
Grassi F, Moretto N, Rivetti C, Cellai S, Betti M, Márquez AJ et al (2006) Structural and functional properties of lengsin, a pseudo-glutamine synthetase in the transparent human lens. Biochem Biophys Res Commun 350:424–429
Greiling TM, Houck SA, Clark JI (2009) The zebrafish lens proteome during development and aging. Mol Vis 15:2313–2125
Greiling TM, Aose M, Clark JI (2010) Cell fate and differentiation of the developing ocular lens. Invest Ophthalmol Vis Sci 51:1540–1546
Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57
Iseri SU, Osborne RJ, Farrall M, Wyatt AW, Mirza G, Nürnberg G et al (2009) Seeing clearly: the dominant and recessive nature of FOXE3 in eye developmental anomalies. Hum Mutat 30:1378–1386
Islam L, Kelberman D, Williamson L, Lewis N, Glindzicz MB, Nischal KK et al (2015) Functional analysis of FOXE3 mutations causing dominant and recessive ocular anterior segment disease. Hum Mutat 36:296–300
Kakrana A, Yang A, Anand D, Djordjevic D, Ramachandruni D, Singh A, Huang H, Ho JWK, Lachke SA (2018) iSyTE 2.0: a database for expression-based gene discovery in the eye. Nucleic Acids Res 46(D1):D875–D885
Kannabiran C, Rogan PK, Olmos L, Basti S, Rao GN, Kaiser-Kupfer M et al (1998) Autosomal dominant zonular cataract with sutural opacities is associated with a splice mutation in the betaA3/A1-crystallin gene. Mol Vis 4:21
Khan AO, Aldahmesh MA, Alkuraya FS (2015) Phenotypes of recessive pediatric cataract in a cohort of children with identified homozygous gene mutations (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 113:T7
Khan SY, Vasanth S, Kabir F, Gottsch JD, Khan AO, Chaerkady R et al (2016) FOXE3 contributes to Peters anomaly through transcriptional regulation of an autophagy-associate protein termed DNAJB1. Nat Commun 7:10953
Kloeckener-Gruissem B, Vandekerckhove K, Nürnberg G, Neidhardt J, Zeitz C, Nürnberg P et al (2008) Mutation of Solute Carrier SLC16A12 Associates with a Syndrome Combining Juvenile Cataract with Microcornea and Renal Glucosuria. Am J Hum Genet 82:772–779
Kuang S-Q, Medina-Martinez O, Guo D, Gong L, Regalado ES, Reynolds CL et al (2016) FOXE3 mutations predispose to thoracic aortic aneurysms and dissections. J Clin Invest 126:948–961
Lachke SA, Ho JW, Kryukov GV, O’Connell DJ, Aboukhalil A, Bulyk ML et al (2012) iSyTE: integrated Systems Tool for Eye gene discovery. Invest Ophthalmol Vis Sci 53:1617–1627
Landgren H, Blixt A, Carlsson P (2008) Persistent FoxE3 expression blocks cytoskeletal remodeling and organelle degradation during lens fiber differentiation. Invest Ophthalmol Vis Sci 49:4269–4277
Medina-Martinez O, Jamrich M (2007) Foxe view of lens development and disease. Development 134:1455–1463
Medina-Martinez O, Brownell I, Amaya-Manzanares F, Hu Q, Behringer RR, Jamrich M (2005) Severe defects in proliferation and differentiation of lens cells in Foxe3 null mice. Mol Cell Biol 25:8854–8863
Nowak RB, Fischer RS, Zoltoski RK, Kuszak JR, Fowler VM (2009) Tropomodulin1 is required for membrane skeleton organization and hexagonal geometry of fiber cells in the mouse lens. J Cell Biol 186:915–928
Ogden AT, Nunes I, Ko K, Wu S, Hines CS, Wang AF et al (1998) GRIFIN, a novel lens-specific protein related to the galectin family. J Biol Chem 273:28889–28896
Ormestad M, Blixt A, Churchill A, Martinsson T, Enerbäck S, Carlsson P (2002) Foxe3 haploinsufficiency in mice: a model for Peters’ anomaly. Invest Ophthalmol Vis Sci 43:1350–1357
Pichi F, Lembo A, Serafino M, Nucci P (2016) Genetics of congenital cataract. Dev Ophthalmol 57:1–14
Reis LM, Tyler RC, Muheisen S, Raggio V, Salviati L, Han DP et al (2013) Whole exome sequencing in dominant cataract identifies a new causative factor, CRYBA2, and a variety of novel alleles in known genes. Hum Genet 132:761–770
Saboo US, Penke D, Mahindrakar A, Uddaraju M, Sankurathri C, Gong X et al (2017) Exome sequencing reveals novel homozygous FOXE3 mutation in microphthalmos with staphylomatous malformation. Ophthalmic Genet 38:295–297
Sarkar PS, Appukuttan B, Han J, Ito Y, Ai C, Tsai W et al (2000) Heterozygous loss of Six5 in mice is sufficient to cause ocular cataracts. Nat Genet 25:110–114
Semina EV, Brownell I, Mintz-Hittner HA, Murray JC, Jamrich M (2001) Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet 10:231–236
Shi X, Luo Y, Howley S, Dzialo A, Foley S, Hyde DR et al (2006) Zebrafish foxe3: roles in ocular lens morphogenesis through interaction with pitx3. Mech Dev 123:761–782
Shi X, Cui B, Wang Z, Weng L, Xu Z, Ma J et al (2009) Removal of Hsf4 leads to cataract development in mice through down-regulation of gamma S-crystallin and Bfsp expression. BMC Mol Biol 10:10
Siddam AD, Gautier-Courteille C, Perez-Campos L, Anand D, Kakrana A, Dang CA, Legagneux V, Méreau A, Viet J, Gross JM, Paillard L, Lachke SA (2018) The RNA-binding protein Celf1 post-transcriptionally regulates p27Kip1 and Dnase2b to control fiber cell nuclear degradation in lens development. PLoS Genet 14(3):e1007278
Somasundaram T, Bhat SP (2004) Developmentally dictated expression of heat shock factors: exclusive expression of HSF4 in the postnatal lens and its specific interaction with alphaB-crystallin heat shock promoter. J Biol Chem 279:44497–44503
Sorokina EA, Muheisen S, Mlodik N, Semina EV (2011) MIP/Aquaporin 0 represents a direct transcriptional target of PITX3 in the developing lens. PLoS One 6:e21122
Steinhart MR, Cone-Kimball E, Nguyen C, Nguyen TD, Pease ME, Chakravarti S et al (2014) Susceptibility to glaucoma damage related to age and connective tissue mutations in mice. Exp Eye Res 119:54–60
Swindell EC, Zilinski CA, Hashimoto R, Shah R, Lane ME, Jamrich M (2008) Regulation and function of foxe3 during early zebrafish development. Genesis 46:177–183
Talbot JC, Amacher SL (2014) A streamlined CRISPR pipeline to reliably generate zebrafish frameshifting alleles. Zebrafish 11:583–585
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-SEq. Bioinformatics 25:1105–1111
Ullah E, Nadeem Saqib MA, Sajid S, Shah N, Zubair M, Khan MA et al (2016) Genetic analysis of consanguineous families presenting with congenital ocular defects. Exp Eye Res 146:163–171
Valleix S, Niel F, Nedelec B, Algros MP, Schwartz C, Delbosc B et al (2006) Homozygous nonsense mutation in the FOXE3 gene as a cause of congenital primary aphakia in humans. Am J Hum Genet 79:358–364
Wada K, Maeda YY, Watanabe K, Oshio T, Ueda T, Takahashi G et al (2011) A deletion in a cis element of Foxe3 causes cataracts and microphthalmia in rct mice. Mamm Genome 22:693–702
Williamson KA, FitzPatrick DR (2014) The genetic architecture of microphthalmia, anophthalmia and coloboma. Eur J Med Genet 57:369–380
Wu D, Mandal S, Choi A, Anderson A, Prochazkova M, Perry H et al (2015) DLX4 is associated with orofacial clefting and abnormal jaw development. Hum Mol Genet 24:4340–4352
Wyatt K, White HE, Wang L, Bateman OA, Slingsby C, Orlova EV, Wistow G (2006) Lengsin is a survivor of an ancient family of class I glutamine synthetases re-engineered by evolution for a role in the vertebrate lens. Structure 14:1823–1834
Yahyavi M, Abouzeid M, Gawdat G, de Preux A-S, Xiao T, Bardakjian T et al (2013) ALDH1A3 loss of function causes bilateral anophthalmia/microphthalmia and hypoplasia of the optic nerve and optic chiasm. Hum Mol Genet 22:3250–3258
Acknowledgements
Library preparation and QCs for sequencing was conducted by Yanxia Hao, Jim McGuire and Natasha Carli, PhD, at the Gladstone Genomics Core.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors have potential conflicts of interest to disclose.
Human rights and animal participants
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
439_2018_1884_MOESM7_ESM.tif
Fig. S1. Chromatograms demonstrating indel variants in F1 fish after CRISPR/Cas9 injections targeting foxe3 (TIF 734 KB)
Rights and permissions
About this article
Cite this article
Krall, M., Htun, S., Anand, D. et al. A zebrafish model of foxe3 deficiency demonstrates lens and eye defects with dysregulation of key genes involved in cataract formation in humans. Hum Genet 137, 315–328 (2018). https://doi.org/10.1007/s00439-018-1884-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-018-1884-1