Abstract
Phytophthora capsici is a hemibiotrophic, phytopathogenic oomycete that infects a wide range of crops, resulting in significant economic losses worldwide. By means of a diverse arsenal of secreted effector proteins, hemibiotrophic pathogens may manipulate plant cell death to establish a successful infection and colonization. In this study, we described the analysis of the gene family encoding necrosis- and ethylene-inducing peptide 1 (Nep1)-like proteins (NLPs) in P. capsici, and identified 39 real NLP genes and 26 NLP pseudogenes. Out of the 65 predicted NLP genes, 48 occur in groups with two or more genes, whereas the remainder appears to be singletons distributed randomly among the genome. Phylogenetic analysis of the 39 real NLPs delineated three groups. Key residues/motif important for the effector activities are degenerated in most NLPs, including the nlp24 peptide consisting of the conserved region I (11-aa immunogenic part) and conserved region II (the heptapeptide GHRHDWE motif) that is important for phytotoxic activity. Transcriptional profiling of eight selected NLP genes indicated that they were differentially expressed during the developmental and plant infection phases of P. capsici. Functional analysis of ten cloned NLPs demonstrated that Pc11951, Pc107869, Pc109174 and Pc118548 were capable of inducing cell death in the Solanaceae, including Nicotiana benthamiana and hot pepper. This study provides an overview of the P. capsici NLP gene family, laying a foundation for further elucidating the pathogenicity mechanism of this devastating pathogen.





Similar content being viewed by others
References
Albert I, Böhm H, Albert M et al (2015) An RLP23–SOBIR1–BAK1 complex mediates NLP-triggered immunity. Nat Plants 1:15140
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Amsellem Z, Cohen BA, Gressel J (2002) Engineering hypervirulence in a mycoherbicidal fungus for efficient weed control. Nat Biotechnol 20:1035–1039
Arenas YC, Kalkman ERIC., Schouten A, Dieho M, Vredenbregt P, Uwumukiza B, Ruiz MO, van Kan JAL (2010) Functional analysis and mode of action of phytotoxic Nep1-like proteins of Botrytis cinerea. Physiol Mol Plant P 74:376–386
Asai S, Shirasu K (2015) Plant cells under siege: plant immune system versus pathogen effectors. Curr Opin Plant Biol 28:1–8
Bae H, Bowers JH, Tooley PW, Bailey BA (2005) NEP1 orthologs encoding necrosis and ethylene inducing proteins exist as a multigene family in Phytophthora megakarya, causal agent of black pod disease on cacao. Mycol Res 109:1373–1385
Bailey BA (1995) Purification of a protein from culture filtrates of Fusarium oxysporum that induces ethylene and necrosis in leaves of Erythroxylum coca. Phytopathology 85:1250–1255
Bailey BA, Apel-Birkhold PC, Luster DG (2002) Expression of NEP1 by Fusarium oxysporum f. sp. erythroxyli after gene replacement and overexpression using polyethylene glycol-mediated transformation. Phytopathology 92:833–841
Baxter L, Tripathy S, Ishaque N et al (2010) Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science 330:1549–1551
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795
Böhm H, Albert I, Oome S, Raaymakers TM, Van den Ackerveken G, Nürnberger T (2014) A conserved peptide pattern from a widespread microbial virulence factor triggers pattern-induced immunity in Arabidopsis. PLoS Pathog 10:e1004491
Bouwmeester K, van Poppel PMJA., Govers F (2009) Genome biology cracks enigmas of oomycete plant pathogens. In: Parker JE (ed) Molecular aspects of plant disease resistance. Wiley-Blackwell, Oxford, pp 102–133
Bozkurt TO, Schornack S, Banfield MJ, Kamoun S (2012) Oomycetes, effectors, and all that jazz. Curr Opin Plant Biol 15:483–492
Cabral A, Oome S, Sander N, Küfner I, Nürnberger T, Van den Ackerveken G (2012) Nontoxic Nep1-like proteins of the downy mil-dew pathogen Hyaloperonospora arabidopsidis: repression of necro-sis-inducing activity by a surface-exposed region. Mol Plant Microbe Interact 25:697–708
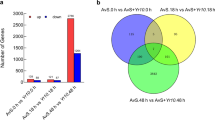
Chen XR, Xing YP, Li YP, Tong YH, Xu JY (2013) RNA-Seq reveals infection-related gene expression changes in Phytophthora capsici. PLoS One 8:e74588
Chen XR, Zhang BY, Xing YP, Li QY, Li YP, Tong YH, Xu JY (2014) Transcriptomic analysis of the phytopathogenic oomycete Phytophthora cactorum provides insights into infection-related effectors. BMC Genom 15:980
Cuomo CA, Guldener U, Xu JR et al (2007) The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317:1400–1402
Dean RA, Talbot NJ, Ebbole DJ et al (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434:980–986
Dong S, Kong G, Qutob D et al (2012) The NLP toxin family in Phytophthora sojae includes rapidly evolving groups that lack necrosis-inducing activity. Mol Plant Microbe Interact 25:896–909
Erwin DC, Ribeiro OK (1996) Phytophthora diseases worldwide. APS, St. Paul
Fellbrich G, Romanski A, Varet A, Blume B, Brunner F, Engelhardt S, Felix G, Kemmerling B, Krzymowska M, Nürnberger T (2002) NLP1, a Phytophthora associated trigger of plant defense in parsley and Arabidopsis. Plant J 32:375–390
Feng BZ, Li PQ (2013) Molecular characterization and functional analysis of the Nep1-like protein-encoding gene from Phytophthora capsici. Genet Mol Res 12:1468–1478
Feng BZ, Li PQ, Fu L, Sun BB, Zhang XG (2011) Identification of 18 genes encoding necrosis-inducing proteins from the plant pathogen Phytophthora capsici (Pythiaceae: Oomycetes). Genet Mol Res 10:910–922
Feng BZ, Zhu XP, Fu L, Lv RF, Storey D, Tooley P, Zhang XG (2014) Characterization of necrosis-inducing NLP proteins in Phytophthora capsici. BMC Plant Biol 14:126
Franceschetti M, Maqbool A, Jiménez-Dalmaroni MJ, Pennington HG, Kamoun S, Banfield MJ (2017) Effectors of filamentous plant pathogens: commonalities amid diversity. Microbiol Mol Biol Rev 81:e00066–e00016
Gijzen M, Nurnberger T (2006) Nep1-like proteins from plant pathogens: recruitment and diversification of the NPP1 domain across taxa. Phytochemistry 67:1800–1807
Haas BJ, Kamoun S, Zody MC et al (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461:393–398
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid Symp Ser 41:95–98
Hein I, Gilroy EM, Armstrong MR, Birch PRJ (2009) The zig–zag–zig in oomycete–plant interactions. Mol Plant Pathol 10:547–562
Jones L, Hamilton AJ, Voinnet O, Thomas CL, Maule AJ, Baulcombe DC (1999) RNA–DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell 11:2291–2301
Judelson HS, Ah-Fong AM, Aux G et al (2008) Gene expression profiling during asexual development of the late blight pathogen Phytophthora infestans reveals a highly dynamic transcriptome. Mol Plant Microbe Interact 21:433–447
Kamoun S (2006) A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol 44:41–60
Kamoun S, van West P, de Jong AJ, de Groot KE, Vleeshouwers VG, Govers F (1997) A gene encoding a protein elicitor of Phytophthora infestans is down-regulated during infection of potato. Mol Plant Microbe Interact 10:13–20
Kamoun S, Furzer O, Jones JD et al (2015) The top 10 oomycete pathogens in molecular plant pathology. Mol Plant Pathol 16:413–434
Kanneganti TD, Huitema E, Cakir C, Kamoun S (2006) Synergistic interactions of the plant cell death pathways induced by Phytophthora infestans Nep1-like protein PiNPP1.1 and INF1 elicitin. Mol Plant Microbe Interact 19:854–863
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lamour KH, Stam R, Jupe J, Huitema E (2012) The oomycete broad-host-range pathogen Phytophthora capsici. Mol Plant Pathol 13:329–337
Larkin MA, Blackshields G, Brown NP et al (2007) ClustalW and ClustalX version 2. Bioinformatics 23:2947–2948
Mattinen L, Tshuikina M, Mae A, Pirhonen M (2004) Identification and characterization of Nip, necrosis-inducing virulence protein of Erwinia carotovora subsp. carotovora. Mol Plant Microbe Interact 17:1366–1375
Motteram J, Küfner I, Deller S, Brunner F, Hammond-Kosack KE, Nürnberger T, Rudd JJ (2009) Molecular characterization and function analysis of MgNLP, the sole NPP1 domain-containing protein, from the fungal wheat leaf pathogen Mycosphaerella graminicola. Mol Plant Microbe Interact 22:790–799
Oh SK, Young C, Lee M et al (2009) In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell 21:2928–2947
Oome S, Van den Ackerveken G (2014) Comparative and functional analysis of the widely occurring family of Nep1-like proteins. Mol Plant Microbe Interact 27:1081–1094
Oome S, Raaymakers TM, Cabral A et al (2014) Nep1-like proteins from three kingdoms of life act as a microbe-associated molecular pattern in Arabidopsis. Proc Natl Acad Sci USA 111:16955–16960
Ottmann C, Luberacki B, Kufner I et al (2009) A common toxin fold mediates microbial attack and plant defense. Proc Natl Acad Sci USA 106:10359–10364
Pemberton CL, Salmond GP (2004) The Nep1-like proteins—a growing family of microbial elicitors of plant necrosis. Mol Plant Pathol 5:353–359
Pemberton CL, Whitehead NA, Sebaihia M et al (2005) Novel quorum-sensing-controlled genes in Erwinia carotovora subsp. carotovora: identification of a fungal elicitor homologue in a soft-rotting bacterium. Mol Plant Microbe Interact 18:343–353
Qutob D, Kamoun S, Gijzen M (2002) Expression of a Phytophthora sojae necrosis-inducing protein occurs during transition from biotrophy to necrotrophy. Plant J 32:361–373
Qutob D, Kemmerling B, Brunner F (2006) Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 18:3721–3744
Santhanam P, van Esse HP, Albert I, Faino L, Nürnberger T, Thomma BP (2013) Evidence for functional diversification within a fungal NEP1-like protein family. Mol Plant Microbe Interact 26:278–286
Seidl MF, Van den Ackerveken G, Govers F, Snel B (2011) A domain-centric analysis of oomycete plant pathogen genomes reveals unique protein organization. Plant Physiol 155:628–644
Staats M, van Baarlen P, Schouten A, van Kan JA (2007) Functional analysis of NLP genes from Botrytis elliptica. Mol Plant Pathol 8:209–214
Thines M, Kamoun S (2010) Oomycete-plant coevolution: recent advances and future prospects. Curr Opin Plant Biol 13:427–433
Tyler BM, Tripathy S, Zhang X et al (2006) Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313:1261–1266
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40:e115
Wang Q, Han C, Ferreira AO et al (2011) Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. Plant Cell 23:2064–2086
Zelaya-Molina LX, Ortega MA, Dorrance AE (2011) Easy and efficient protocol for oomycete DNA extraction suitable for population genetic analysis. Biotechnol Lett 33:715–720
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31671971), Natural Science Foundation of Yangzhou City (China) (no. YZ2016121), the Special Fund for Agro-Scientific Research in the Public Interest of China (no. 201303018) and the Yangzhou University 2016 Project for Excellent Young Key Teachers. The authors thank Dr. Paul Morris (Bowling Green State University, OH, USA) for his critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by S. Hohmann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
438_2018_1432_MOESM4_ESM.docx
Supplementary material 4 Online Resource 4 Phylogenetic relationship of 10 NLPs cloned from P. capsici and 7 other microorganism-derived NLPs. The analysis was performed using the maximum likelihood algorithm in MEGA7. Percent bootstrap values (1000 replicates) are shown above the forks. Accession numbers of species: AAM48170 (PsojNIP, P. sojae), AEZ06585 (HaNLP9, H. arabidopsidis), AAS45247 (VdNLP, V. dahlia,), XP_365630 (MoNLP, M. oryzae), CAF05864 (NcNLP, N. crassa), XP_748132 (AfNLP, A. fumigatus) and ELR02678 (PdNLP, P. destructans). The scale bar shows expected changes per site (DOCX 23 KB)
Rights and permissions
About this article
Cite this article
Chen, XR., Huang, SX., Zhang, Y. et al. Identification and functional analysis of the NLP-encoding genes from the phytopathogenic oomycete Phytophthora capsici. Mol Genet Genomics 293, 931–943 (2018). https://doi.org/10.1007/s00438-018-1432-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-018-1432-7