Abstract
Human trichinellosis is acquired by eating raw or undercooked meats carrying muscle larvae of Trichinella spp. Toll-like receptors (TLRs) are essential components of the innate immune system. However, little is known about the potential application of TLR agonists for immunotherapy against Trichinella spiralis (T. spiralis) infection. Here, we evaluated the effects of four TLR agonists (i.e., TLR3, TLR4, TLR8, and TLR9 agonists) on T. spiralis infection in mice. The reduction rate of worm burden showed that TLR3 agonist poly(I:C) significantly reduced T. spiralis infection rather than TLR4, TLR8, and TLR9 agonists (p < 0.05). Moreover, TLR3 showed a continuous high-level of expression during 6–35 days post infection (dpi). The levels of interferon-gamma (IFN-γ), interleukin (IL)-2, and IL-6 increased significantly in mice serum compared with control group after treatment with TLR3 agonist at 0, 3, 6, 9, 12, 15, 18, 21, 28, and 35 dpi (p < 0.05). A significant decreasing trend was also detected in levels of IL-10 and IL-4 after treatment with TLR3 agonist compared with control group at 0, 3, 6, 9, 12, 15, 18, 21, 28, and 35 dpi (p < 0.05). Overall, this study suggested that TLR3-targeted therapies might be effective on worm burden reduction by regulation of the cytokine levels in the mice infected with T. spiralis.
Similar content being viewed by others
Introduction
Human trichinellosis has been listed as a neglected tropical disease, reported in 55 countries worldwide, and seriously affected the public health, slaughtering industries as well as the import and export trade (Pozio 2007; Shimoni and Froom 2015; Wang et al. 2012). The severity of human trichinellosis can range from subclinical to fatal (Gomez-Morales et al. 2018). Indeed, the early diagnosis and treatment of trichinellosis are of great importance in reducing major public health risk.
The genus Trichinella is a unique nematode that the adult worms and the larvae parasitize in the same host (Ashour 2013). Trichinella spiralis (T. spiralis) infection begins when larvae are released from cysts and invade the small intestine, and then the larvae mature into adult worms. After copulation, the female adult worms release newborn larvae which travel through the circulatory system to skeletal muscle cells where they encyst and develop into muscle larvae (Lee and Best 1983). Toll-like receptors (TLRs) are widely expressed in various immune cells, including dendritic cells, macrophages, B cells, specific types of T cells, and even in non-immune cells, such as epithelial, endothelial, and fibroblast cells (Lester and Li 2014; Manicassamy and Pulendran 2009; Sun et al. 2011; Zakeri et al. 2016). TLR protective immunity against some parasitic helminths conferred by T helper type 1 or 2 (Th1 or Th2, respectively) cytokine responses (Perrigoue et al. 2008). During the intestinal phase, the immune response is mixed, and the Th1 response characterized by increased interferon gamma (IFN-γ), interleukin(IL)-2, and IL-12 was predominant at the initial stage, and the Th2 response characterized by increased IL-4, IL-5, IL-9, IL-10, and IL-13 was elevated subsequently (Aranzamendi et al. 2012; Ding et al. 2017; Ishikawa et al. 1998). The expulsion of T. spiralis is dependent upon the polarized Th2 immune response regulated by TLRs (Nutman 2015). In fact, downstream TLRs negatively correlated with the development of Th2 cytokine responses of parasitic helminths (Perrigoue et al. 2008). In mouse embryonic fibroblast cells, TLR4 and TLR9 expression was significantly increased and TLR3 expression significantly decreased after treatment with ES protein of T. spiralis (Kim et al. 2015). Therefore, a dual effect on TLRs could be exerted by activation or negative regulation of these receptors by T. spiralis (Ilic et al. 2012). The study of TLRs should shed more light on the role played by TLRs dysregulation and provide new application studies for therapeutic strategy and vaccine development (Venugopal et al. 2009). TLR immunotherapy can prevent antigen-specific and independent generalized immunosuppression in a susceptible population (Dasgupta et al. 2014). Further studies are needed for the screening of potential TLR agonists for immunotherapy against T. spiralis infection.
Material and methods
Animals and parasites
T. spiralis stains were identified by OIE Collaborating Center on Foodborne Parasites in Asian-Pacific Region in August 2014 and maintained in Institute of Zoonoses, Jilin University. Female C57BL/6 mice, 6–8 weeks old, were purchased from Jilin University experimental animal center (Jilin, China), with the certificate No. 2016–0001 in conformity of SCXK (Jilin). The muscle larvae of T. spiralis were recovered from infected mice by artificial digestion method as previously described (Friend et al. 1996).
Infection of T. spiralis and determination of parasite burden
All mice were infected with 300 muscle larvae per os. Experimental mice infected with T. spiralis were given the reported TLR3 agonist poly(I:C) (InvivoGen) 7.5 mg/kg, TLR4 agonist LPS (InvivoGen) 2 mg/kg, TLR8 agonist TL8-506 (InvivoGen) 5 mg/kg, and TLR9 agonist ONDM362 (InvivoGen) 5 mg/kg by tail vein injection at 0, 2, 5, 8, 11, 14, 17, 20, and 27, 34 days post infection (dpi). Meanwhile, the control group of mice was only injected with PBS. Parasite burden was calculated as the total number of larvae per gram (LPG) of muscle tissue at 35 dpi. The reduction percentage of LPG in the treated animals was calculated as follows: LPG reduction rate = (control group mean LPG − treated group mean LPG) / control group mean LPG × 100% (Garcia et al. 2013; Gurish et al. 2004)
Experimental infection and treatment with poly(I:C)
Two groups of 10 mice were infected with 300 muscle larvae of T. spiralis (T1, ISS534) per os. The experimental mice infected with T. spiralis were given poly(I:C) 300 μg per mouse by tail vein injection at 0, 2, 5, 8, 11, 14, 17, 20, 27, and 34 dpi. The serum was collected from each mouse at 0, 3, 6, 9, 12, 15, 18, 21, 28, and 35 dpi (Bruschi et al. 2014).
Real-time PCR
Total RNA was extracted from splenocyte with Trizol reagent (Invitrogen) at 0, 3, 6, 9, 12, 15,18, 21, 28, and 35 dpi. In total, 1 μg RNA was reverse transcribed to cDNA using RNA simple total RNA Kit according to the manufacturer’s instructions (Tiangen Biochemical Technology). Real-time PCR experiments were carried out in triplicate with SYBR premix Ex Taq™ II (Promega) on an ABI 7500 Fast Real-Time PCR System (ABI). The PCR primers were designed by the Primer-BLAST tool. TLR3 primers: forward primer 5′- caaaccccggtggtcccgtt-3′ and reverse primer 5′- aaggcggcccgaaaacatcct-3′. For normalization of target gene expression, glyceraldehyde-3-phosphate dehydrogenase (GAPDH forward primer 5′-atgacatcaagaaggtggtgaag-3′ and reverse primer 5′-tccttggaggccatgtagg-3') was taken for the calculation of a reference gene. Relative mRNA expression was analyzed by using the Applied Biosystems 7500 Software and calculated by the comparative Ct method (Schmittgen and Livak 2008).
ELISA
Serum samples were collected from the mice in control and treated group at 0, 3, 6, 9, 12, 15, 18, 21, 28, 35 dpi. Serum cytokine was assayed for levels of IFN-γ, IL-2, IL-4, IL-6, and IL-10 using ELISA Kit (eBioscience) (Mansson Kvarnhammar et al. 2013).
Statistics
The results are expressed as the mean ± standard error, SE. Comparisons between control and treated groups were performed with paired-samples t tests by SPSS 10.0 software (SPSS Inc., Chicago, IL, USA). Differences were considered significant at a 5% significance level (p < 0.05).
Results
Effects of TLR agonists on parasite burden
Parasite burdens of muscle larvae were compared among the groups which were given with poly(I:C), LPS, TL8-506, ONDM362, respectively, and control group. The maximum reduction rate of parasite burden was observed in poly(I:C) treatment group (p < 0.05) (Table 1).
Expression of TLR3 in splenocyte
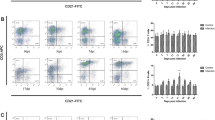
The statistical analysis of TLR3 expression in spleen between control group and poly(I:C) treatment group during 0–3 dpi showed no significant differences. Compared to control group, TLR3 exhibited a continuous high-level expression in poly(I:C) treatment group during 6–35 dpi (Fig. 1).
Cytokine production stimulated by poly(I:C) treatment
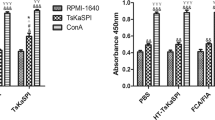
Th1 cytokines (i.e., IFN-γ, IL-2) and Th2 cytokine IL-6 in mice serum significantly increase after poly(I:C) treatments at 0, 3, 6, 9, 12, 15, 18, 21, 28, and 35 dpi compared with control group (p < 0.05; Fig. 2 a, b, and c). Significant decreases in levels of Th2 cytokines (i.e., IL-4 and IL-10) were detected in control and treatment group at 0, 3, 6, 9, 12, 15, 18, 21, 28, and 35 dpi (Fig. 2d, e; p < 0.05).
The level of cytokine production in serum after stimulation with poly(I:C). The serum samples were collected from mice infected with T. spiralis after poly(I:C) and PBS treatment (n = 10). The production levels of IFN-γ (a), IL-2 (b), IL-6 (c), IL-4 (d), and IL-10 (e) were detected at 0, 3, 6, 9, 12, 15, 18, 21, 28, and 35 days post infection.
Discussion
TLRs represent one of the most studied pattern recognition receptor (PRR) families (Harris et al. 2006; Tartey and Takeuchi 2017). Qu demonstrated that TLR3 is the primary molecule which modulates the activation and function of NK cells during Schistosoma japonicum (S. japonicum) infection in mice (Qu et al. 2018). The expression levels of TLR3 on different pulmonary lymphocytes were increased after S. japonicum infection (Chen et al. 2019). T. spiralis can also modulate the immune response by regulating the expression of TLRs and their signaling pathways (Yu et al. 2013). Here, we analyzed the effects of TLR agonist on mice infected with T. spiralis. After treatment, the TLR3 agonist poly(I:C) was considered as the best candidate among the four kinds of TLR agonists. Our study provided evidence that the TLR3 agonist was able to interfere with the parasitism of T. spiralis. According to our results, experimental mice infected with T. spiralis after poly(I:C) treatment showed a consistently high-level expression of TLR3 comparing to that in control group during 6–35 dpi. These results indicated that TLR3 agonists could activate TLR3 at different stages of T. spiralis infection and suggested that TLR3 agonists might affect the infection of T. spiralis. The activation of TLRs promotes both innate immunity responses and the induction of adaptive immunity (Cui et al. 2014). As Toll-like receptor expression level changes during T. spiralis infection, Th1 and Th2 responses could be regulated and induce T lymphocyte proliferation and cytokine production which also affected the function of antigen-presenting cells (Brown et al. 2014; Mansson Kvarnhammar et al. 2013). In the previous study, it is indicated that T. spiralis can induce a complex Th1/Th2 response with predominant polarization to Th2 during intestinal and muscle phase (Ding et al. 2017). As reported in the “Material and methods” section, the expression levels of IFN-γ, IL-2, IL-4, IL-6, and IL-10 were detected by ELISA. We observed that T. spiralis could regulate the cytokine expression of the host at different stages. The proinflammatory cytokines (i.e., Th1 type cytokines IFN-γ, IL-2, and Th2 type cytokine IL-6) in mice significantly increased after poly(I:C) treatment compared with control group (Fig. 2 a, b, and c). Meanwhile, significant decreases of the anti-inflammatory cytokines (i.e. Th2 cytokines IL-4 and IL-10) were observed in experimental group (Fig. 2d, e; p < 0.05). Therefore, the reduction of the parasite burden may be related to these proinflammatory responses caused by Th1 cytokine increase. Indeed, many preclinical and clinical studies have demonstrated that the use of purified TLR agonists or TLR ligands as adjuvants represents a considerable potential TLR-targeted therapy against a variety of inflammatory diseases and autoimmune conditions (Kamdar et al. 2013; Majewska and Szczepanik 2006). It is clear that TLR agonists can be useful for several purposes, including signal therapy to accelerate and enhance the induction of vaccine-specific responses (Dowling and Mansell 2016). Above all, TLRs could be considered as a “Swiss Army” knife of the immune system with the capability of applying to the development of vaccines against trichinellosis. In this study, we evaluated the effects of four TLR agonists on T. spiralis infection in mice and found that TLR3 agonist administration could reduce the parasite burden significantly, and the levels of the proinflammatory cytokines (IFN-γ, IL-2, and IL-6) increase and the levels of anti-proinflammatory cytokines (IL-4 and IL-10) decrease after TLR3 agonist treatment.
References
Aranzamendi C et al (2012) Trichinella spiralis-secreted products modulate DC functionality and expand regulatory T cells in vitro. Parasite Immunol 34:210–223. https://doi.org/10.1111/j.1365-3024.2012.01353.x
Ashour DS (2013) Trichinella spiralis immunomodulation: an interactive multifactorial process. Expert Rev Clin Immunol 9:669–675. https://doi.org/10.1586/1744666X.2013.811187
Brown M, Hughes KR, Moossavi S, Robins A, Mahida YR (2014) Toll-like receptor expression in crypt epithelial cells, putative stem cells and intestinal myofibroblasts isolated from controls and patients with inflammatory bowel disease. Clin Exp Immunol 178:28–39. https://doi.org/10.1111/cei.12381
Bruschi F, Bianchi C, Fornaro M, Naccarato G, Menicagli M, Gomez-Morales MA, Pozio E, Pinto B (2014) Matrix metalloproteinase (MMP)-2 and MMP-9 as inflammation markers of Trichinella spiralis and Trichinella pseudospiralis infections in mice. Parasite Immunol 36:540–549. https://doi.org/10.1111/pim.12138
Chen D, Zhao Y, Feng Y, Jin C, Yang Q, Qiu H, Xie H, Xie S, Zhou Y, Huang J (2019) Expression of TLR2, TLR3, TLR4, and TLR7 on pulmonary lymphocytes of Schistosoma japonicum-infected C57BL/6 mice. Innate Immun 25:224–234. https://doi.org/10.1177/1753425919840424
Cui J, Chen Y, Wang HY, Wang RF (2014) Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum Vaccin Immunother 10:3270–3285. https://doi.org/10.4161/21645515.2014.979640
Dasgupta S, Aghazadeh-Dibavar S, Bandyopadyay M (2014) The role of toll-like receptor agonists in the immunotherapy of leishmaniosis. An update and proposal for a new form of anti-leishmanial therapy. Ann Parasitol 60:75–82
Ding J, Bai X, Wang X, Shi H, Cai X, Luo X, Liu M, Liu X (2017) Immune cell responses and cytokine profile in intestines of mice infected with Trichinella spiralis. Front Microbiol 8:2069. https://doi.org/10.3389/fmicb.2017.02069
Dowling JK, Mansell A (2016) Toll-like receptors: the swiss army knife of immunity and vaccine development. Clin Transl Immunology 5:e85. https://doi.org/10.1038/cti.2016.22
Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL (1996) Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol 135:279–290. https://doi.org/10.1083/jcb.135.1.279
Garcia A et al (2013) Novel albendazole formulations given during the intestinal phase of Trichinella spiralis infection reduce effectively parasitic muscle burden in mice. Parasitol Int 62:568–570. https://doi.org/10.1016/j.parint.2013.08.009
Gomez-Morales MA, Ludovisi A, Amati M, Cherchi S, Tonanzi D, Pozio E (2018) Differentiation of Trichinella species (Trichinella spiralis/Trichinella britovi versus Trichinella pseudospiralis) using western blot. Parasit Vectors 11:631. https://doi.org/10.1186/s13071-018-3244-3
Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC (2004) IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol 172:1139–1145. https://doi.org/10.4049/jimmunol.172.2.1139
Harris G, KuoLee R, Chen W (2006) Role of toll-like receptors in health and diseases of gastrointestinal tract. World J Gastroenterol 12:2149–2160. https://doi.org/10.3748/wjg.v12.i14.2149
Ilic N, Gruden-Movsesijan A, Sofronic-Milosavljevic L (2012) Trichinella spiralis: shaping the immune response. Immunol Res 52:111–119. https://doi.org/10.1007/s12026-012-8287-5
Ishikawa N, Goyal PK, Mahida YR, Li KF, Wakelin D (1998) Early cytokine responses during intestinal parasitic infections. Immunology 93:257–263. https://doi.org/10.1046/j.1365-2567.1998.00412.x
Kamdar K, Nguyen V, DePaolo RW (2013) Toll-like receptor signaling and regulation of intestinal immunity. Virulence 4:207–212. https://doi.org/10.4161/viru.23354
Kim S, Park MK, Yu HS (2015) Toll-like receptor gene expression during Trichinella spiralis infection. The Korean journal of parasitology 53:431–438. https://doi.org/10.3347/kjp.2015.53.4.431
Lee CM, Best Y (1983) Immunobiology of trichinosis. J Natl Med Assoc 75:565–570
Lester SN, Li K (2014) Toll-like receptors in antiviral innate immunity. J Mol Biol 426:1246–1264. https://doi.org/10.1016/j.jmb.2013.11.024
Majewska M, Szczepanik M (2006) The role of toll-like receptors (TLR) in innate and adaptive immune responses and their function in immune response regulation. Postepy Hig Med Dosw (Online) 60:52–63
Manicassamy S, Pulendran B (2009) Modulation of adaptive immunity with toll-like receptors. Semin Immunol 21:185–193. https://doi.org/10.1016/j.smim.2009.05.005
Mansson Kvarnhammar A, Tengroth L, Adner M, Cardell LO (2013) Innate immune receptors in human airway smooth muscle cells: activation by TLR1/2, TLR3, TLR4, TLR7 and NOD1 agonists. PLoS One 8:e68701. https://doi.org/10.1371/journal.pone.0068701
Nutman TB (2015) Looking beyond the induction of Th2 responses to explain immunomodulation by helminths. Parasite Immunol 37:304–313. https://doi.org/10.1111/pim.12194
Perrigoue JG, Marshall FA, Artis D (2008) On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell Microbiol 10:1757–1764. https://doi.org/10.1111/j.1462-5822.2008.01174.x
Pozio E (2007) World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol 149:3–21. https://doi.org/10.1016/j.vetpar.2007.07.002
Qu J, Li L, Xie H, Zhang X, Yang Q, Qiu H, Feng Y, Jin C, Dong N, Huang J (2018) TLR3 modulates the response of NK cells against Schistosoma japonicum. J Immunol Res 2018:7519856–7519811. https://doi.org/10.1155/2018/7519856
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Shimoni Z, Froom P (2015) Uncertainties in diagnosis, treatment and prevention of trichinellosis. Expert Rev Anti-Infect Ther 13:1279–1288. https://doi.org/10.1586/14787210.2015.1075394
Sun S, Wang XL, Wu XP, Zhao Y, Wang F, Liu XL, Song YX, Wu ZL, Liu MY (2011) Toll-like receptor activation by helminths or helminth products to alleviate inflammatory bowel disease. Parasit Vectors 4:186. https://doi.org/10.1186/1756-3305-4-186
Tartey S, Takeuchi O (2017) Pathogen recognition and toll-like receptor targeted therapeutics in innate immune cells. Int Rev Immunol 36:57–73. https://doi.org/10.1080/08830185.2016.1261318
Venugopal PG, Nutman TB, Semnani RT (2009) Activation and regulation of toll-like receptors (TLRs) by helminth parasites. Immunol Res 43:252–263. https://doi.org/10.1007/s12026-008-8079-0
Wang ZQ, Li LZ, Jiang P, Liu LN, Cui J (2012) Molecular identification and phylogenetic analysis of Trichinella isolates from different provinces in mainland China. Parasitol Res 110:753–757. https://doi.org/10.1007/s00436-011-2549-3
Yu YR, Deng MJ, Lu WW, Jia MZ, Wu W, Qi YF (2013) Systemic cytokine profiles and splenic toll-like receptor expression during Trichinella spiralis infection. Exp Parasitol 134:92–101. https://doi.org/10.1016/j.exppara.2013.02.014
Zakeri A, Borji H, Haghparast A (2016) Interaction between Helminths and toll-like receptors: possibilities and potentials for asthma therapy. Int Rev Immunol 35:219–248. https://doi.org/10.3109/08830185.2015.1096936
Acknowledgments
We would like to thank OIE Collaborating Center on Foodborne Parasites in Asian-Pacific Region for providing knowledge instruction in detail.
Funding
This work was funded by grants from National Key Research and Development Program of China (2016YFD0500707).
Author information
Authors and Affiliations
Contributions
Bin Tang and Xue Bai performed parasite burden determination. Xiaolei Liu performed ELISA; Yang Wang performed real-time PCR; Jing Ding performed T. spiralis infection and treatment with poly(I:C), and Mingyuan Liu analyzed the data. Xuelin Wang conceived of or designed the study. Xuelin Wang and Bin Tang wrote the paper. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
All the animal husbandry and experimental procedures were performed in accordance with the Chinese Animal Management Ordinance (People’s Republic of China Ministry of Health Document No. 55 in 2001). All the experimental procedures were reviewed and approved by the ethical committee in Jilin University for the care and use of laboratory animals.
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Handling Editor: Una Ryan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, B., Liu, X., Liu, M. et al. Effects of TLR agonists on immune responses in Trichinella spiralis infected mice. Parasitol Res 119, 2505–2510 (2020). https://doi.org/10.1007/s00436-020-06747-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06747-8