Abstract
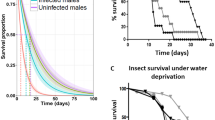
Relatively little is known about the fitness effects and life history trade-offs in medically important parasites and their insect vectors. One such case is the triatomine bugs and the parasite Trypanosoma cruzi, the key actors in Chagas disease. Previous studies have revealed some costs but have not simultaneously examined traits related to development, reproduction, and survival or their possible trade-offs. In addition, these studies have not compared the effects of genetically different T. cruzi strains that differ in their weakening effects in their vertebrate hosts. We compared the body size of the bugs after infection, the number of eggs laid, hatching/non-hatching rate, hatching success, survival, and the resulting number of parasites in Meccus (Triatoma) pallidipennis bugs that were experimentally infected with two strains of T. cruzi (Chilpancingo [CH], the most debilitating in vertebrates; and Morelos [MO], the least debilitating) (both belonging to TcI group). Our results showed that infection affects size (MO < CH; MO and CH = control), number of eggs laid (MO and CH < control) hatching/non-hatching rate (MO < control < CH), hatching success (control < MO, CH = control = MO), and survival (Chilpancingo < Morelos < control). In addition, the CH strain produced more parasites than the MO strain. These results suggest that (a) infection costs depend on the parasite’s origin, (b) the more debilitating effects of the CH strain are due to its increased proliferation in the host, and (c) differences in pathogenicity among T. cruzi strains can be maintained through their different effects on hosts’ life history traits. Probably, the vectorial capacity mediated by a more aggressive strain could be reduced due to its costs on the triatomine, leading to a lower risk of vertebrate and invertebrate infection in natural populations.




Similar content being viewed by others
References
Agnew P, Koella JC, Michalakis Y (2000) Host life history responses to parasitism. Microbes Infect 2:891–896
Bara J, Rapti Z, Cáceres CE, Muturi EJ (2015) Effect of larval competition on extrinsic incubation period and vectorial capacity of Aedes albopictus for dengue virus. PLoS One 10:e0126703
Botto-Mahan C, Cattan PE, Medel R (2006) Chagas disease parasite induces behavioural changes in the kissing bug Mepraia spinolai. Acta Trop 98:219–223. https://doi.org/10.1016/j.actatropica.2006.05.005
Ciota AT, Styer LM, Meola MA, Kramer LD (2011) The costs of infection and resistance as determinants of West Nile virus susceptibility in Culex mosquitoes. BMC Ecol 11:23. https://doi.org/10.1186/1472-6785-11-23
Dolgova O, Lao O (2019) Medicine in the light of evolution. Genes 10:E3. https://doi.org/10.3390/genes10010003
De Fuentes-Vicente JA, Gutiérrez-Cabrera AE, Flores-Villegas AL, Lowenberger C, Benelli G, Salazar-Schettino PM, Córdoba-Aguilar A (2018) What makes an effective Chagas disease vector? Factors underlying Trypanosoma cruzi-triatomine interactions. Acta Trop 183:23–31
De Oliveira ABB, Alevi KCC, Imperador CHL, Madeira FF, Azeredo-Oliveira MTV (2018) Parasite–vector interaction of Chagas disease: a mini-review. Am J Trop Med Hyg 98:653–655
Elliot SL, Rodrigues JO, Lorenzo MG, Martins-Filho OA, Guarneri AA (2015) Trypanosoma cruzi, etiological agent of Chagas disease, is virulent to its triatomine vector Rhodnius prolixus in a temperature-dependent manner. PLoS Negl Trop Dis 9:e0003646. https://doi.org/10.1371/journal.pntd.0003646
Favila-Ruiz G, Jiménez-Cortés JG, Córdoba-Aguilar A, Salazar-Schettino PM, Gutiérrez-Cabrera AE, Pérez-Torres A, De Fuentes-Vicente JA, Vences-Blanco MO, Bucio-Torres M, Flores-Villegas AL, Cabrera-Bravo M (2018) Effects of Trypanosoma cruzi on the phenoloxidase and prophenoloxidase activity in the vector Meccus pallidipennis (Hemiptera: Reduviidae). Parasit Vectors 11:434. https://doi.org/10.1186/s13071-018-3016-0
Fellet MR, Lorenzo MG, Elliot SL, Carrasco D, Guarneri AA (2014) Effects of infection by Trypanosoma cruzi and Trypanosoma rangeli on the reproductive performance of the vector Rhodnius prolixus. PLoS One 9:e105255. https://doi.org/10.1371/journal.pone.0105255
Ferreira RC, Kessler RL, Lorenzo MG, Paim RMM, Ferreira LDL, Probst CM, Guarneri AA (2016) Colonization of Rhodnius prolixus gut by Trypanosoma cruzi involves an extensive parasite killing. Parasitology 143:434–443
Flores-Ferrer A, Marcou O, Waleckx E, Dumontei E, Gourbière S (2018) Evolutionary ecology of Chagas disease; what do we know and what do we need? Evol Appl 11:470–487
Flores-Villegas AL, Cabrera-Bravo M, Toriello C, Bucio-Torres MI, Salazar-Schettino PM, Córdoba-Aguilar A (2016) Survival and immune response of the Chagas vector Meccus pallidipennis (Hemiptera: Reduviidae) against two entomopathogenic fungi, Metarhizium anisopliae and Isaria fumosorosea. Parasit Vectors 9:176. https://doi.org/10.1186/s13071-016-1453-1
Germano MD, Picollo MI (2014) Reproductive and developmental costs of deltamethrin resistance in the Chagas disease vector Triatoma infestans. J Vector Ecol 40:59–65
Gutiérrez-Cabrera AE, Alejandre-Aguilar R, Hernández-Martínez S, Espinoza B (2014) Development and glycoprotein composition of the perimicrovillar membrane in Triatoma (Meccus) pallidipennis (Hemiptera: Reduviidae). Arthropod Struct Dev 43:571–578
Gwynn DM, Callaghan A, Gorham J, Walters KFA, Fellowes MDE (2005) Resistance is costly: trade-offs between immunity, fecundity and survival in the pea aphid. Proc Biol Sci 272:1803–1808
Hinestroza G, Ortiz MI, & Molina J (2016) Behavioral fever response in Rhodnius prolixus (Reduviidae: Triatominae) to intracoelomic inoculation of Trypanosoma cruzi. Rev Soc Bras Med Trop 49:425–432
Hurd H (2003) Manipulation of medically important insect vectors by their parasites. Annu Rev Entomol 48:141–161
Jiménez P, Jaimes J, Poveda C, Ramírez JD (2018) A systematic review of the Trypanosoma cruzi genetic heterogeneity, host immune response and genetic factors as plausible drivers of chronic chagasic cardiomyopathy. Parasitology 13:1–15
Kalinda C, Chimbari M, Mukaratirwa S (2017) Implications of changing temperatures on the growth, fecundity and survival of intermediate host snails of schistosomiasis: a systematic review. Int J Environ Res Public Health 14:80. https://doi.org/10.3390/ijerph14010080
Kaltz O, Shykoff JA (1998) Local adaptation in host–parasite systems. Heredity 81:361–370
Kawecki TJ (1998) Red queen meets Santa Rosalia: arms races and the evolution of host specialization in organisms with parasitic lifestyles. Am Nat 152:635–651
Keymer AE (1980) The influence of Hymenolepis diminuta on the survival and fecundity of the intermediate host, Tribolium confusum. Parasitology 81:405–421
Kollien A, Schaub G (2000) The development of Trypanosoma cruzi in triatominae. Parasitol Today 16:381–387
Kramer LD, Ciota AT (2015) Dissecting vectorial capacity for mosquito-borne viruses. Curr Opin Virol 15:112–118
Lambrechts L, Fellous S, Koella JC (2006) Coevolutionary interactions between host and parasite genotypes. Trends Parasitol 22:12–16
Macedo AM, Pena SDJ (1998) Genetic variability of Trypanosoma cruzi: implications for the pathogenesis of Chagas disease. Parasitol Today 14:119–124
Macedo AM, Oliveira RP, Pena SD (2002) Chagas disease: role of parasite genetic variation in pathogenesis. Expert Rev Mol Med 4:1–16. https://doi.org/10.1017/S1462399402004118
Macedo AM, Machado CR, Oliveira RP, Pena SD (2004) Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem Inst Oswaldo Cruz 99:1–12
Mahmud R, Lim YAL, Amir A (2018) Medical parasitology: a textbook. Springer
Menu F, Ginoux M, Rajon E, Lazzari CR, Rabinovich JE (2010) Adaptive developmental delay in Chagas disease vectors: an evolutionary ecology approach. PLoS Negl Trop Dis 4:e691. https://doi.org/10.1371/journal.pntd.0000691
Paaijmans KP, Blanford S, Chan BH, Thomas MB (2011) Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol Lett 8:465–468
Peterson JK, Graham AL, Dobson AP, Chávez OT (2015) Rhodnius prolixus life history outcomes differ when infected with different Trypanosoma cruzi I strains. Am J Trop Med Hyg 93:564–572
Peterson JK, Graham AL, Elliott RJ, Dobson AP, Chávez OT (2016) Trypanosoma cruzi–Trypanosoma rangeli co-infection ameliorates negative effects of single trypanosome infections in experimentally infected Rhodnius prolixus. Parasitology 143:1157–1167
Poulin R (2007) Evolutionary ecology of parasites. Princeton University Press, Princeton
Råberg L, Graham AL, Read AF (2008) Decomposing health: tolerance and resistance to parasites in animals. Phil Trans R Soc B Biol Sci 364:37–49. https://doi.org/10.1098/rstb.2008.0184
Schaub GA (1988) Developmental time and mortality of larvae of Triatoma infestans infected with Trypanosoma cruzi. Trans R Soc Trop Med Hyg 82:94–96
Schmid-Hempel P (2011). Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. Oxford University Press
Schwartz A, Koella JC (2001) Trade-offs, conflicts of interest and manipulation in Plasmodium–mosquito interactions. Trends Parasitol 17:189–194
Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321
Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B (1996) DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol 83:141–152. https://doi.org/10.1016/S0166-6851(96)02755-7
Téllez–García AA, Bello-Bedoy R, Enríquez-Vara JN, Córdoba–Aguilar A, Gutiérrez–Cabrera AE (In press) Genital morphology and copulatory behavior in triatomine bugs (Reduviidae: Triatominae). Arthropod Struct Dev. https://doi.org/10.1016/j.asd.2018.11.012
Thompson JN (2005) Coevolution: the geographic mosaic of coevolutionary arms races. Curr Biol 15:R992–R994. https://doi.org/10.1016/j.cub.2005.11.046
Tripet F, Aboagye-Antwi F, Hurd H (2008) Ecological immunology of mosquito–malaria interactions. Trends Parasitol 24:219–227
Vézilier J, Nicot A, Gandon S, Rivero A (2012) Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc Biol Sci 279:4033–4041
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100:687–690. https://doi.org/10.1086/282461
Zeledón R, Guardia VM, Zúñiga A, Swartzwelde JC (1970) Biology and ethology of Triatoma dimidiata (Latreille, 1811). II. Life span of adults and fecundity and fertility of females. J Med Entomol 7:462–469
Zingales B (2018) Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop 184:38–52. https://doi.org/10.1016/j.actatropica.2017.09.017
Funding
This study was funded by DGAPA-PAPIIT IN206618.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Section Editor: Marta Teixeira
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary material
Fig. 1. Trypanosoma cruzi genotyping showing a 350 bp indicative of TcI group in both strains. 100 bp molecular weight ladder with size in bp is indicated on the left. Mor: Morelos strain; Ch: Chilpancingo strain; and, C-: template control with no DNA. (PNG 239 kb)
Rights and permissions
About this article
Cite this article
Cordero-Montoya, G., Flores-Villegas, A.L., Salazar-Schettino, P.M. et al. The cost of being a killer’s accomplice: Trypanosoma cruzi impairs the fitness of kissing bugs. Parasitol Res 118, 2523–2529 (2019). https://doi.org/10.1007/s00436-019-06413-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06413-8