Abstract
The Eurasian lynx (Lynx lynx) is a strictly protected species of large carnivore in Poland. It inhabits forest complexes in north-eastern Poland and the Carpathian region in southern Poland. The status of the lynx within Poland requires special attention because its range decreased between 1980 and 2001 and has not yet recovered. One of the factors negatively affecting lynx populations is diseases, particularly parasites. The helminth fauna of the Eurasian lynx is not fully known in Poland. Previous coprological studies revealed that Polish lynx have been infected with seven species of nematodes, three species of cestodes, and one species of trematode. In this study, we present new data based on examination of opportunistically collected lynx carcasses. The aim of the study was to complement data on the helminth fauna of Eurasian lynx inhabiting Poland based on morphological and molecular analysis of parasites. Four species of cestodes—Taenia lynciscapreoli, Mesocestoides lineatus, Spirometra sp., and Taenia krabbei—were found for the first time in Eurasian lynx from Poland and three previously reported species of nematodes—Ancylostoma tubaeforme, Toxascaris leonina, and Toxocara cati—were confirmed. Larvae of Trichinella britovi were also detected in Eurasian lynx in Poland for the first time.
Similar content being viewed by others
Introduction
The Eurasian lynx (Lynx lynx) is a strictly protected large carnivore species in Poland. Despite protection and a general increasing trend in other countries (Chapron et al. 2014), its population within Poland decreased dramatically between 1980 and 2001 (Jędrzejewski et al. 2002) and has not yet recovered. Habitat fragmentation in central Europe has been suggested as a major cause of this situation due to a strong affiliation between these felids and forest habitat (Kowalczyk et al. 2015; Podgórski et al. 2008; Schmidt 1998). Infestation with parasites has been shown to be an important mortality factor in lynx (Breitenmoser et al. 2000; Schmidt-Posthaus et al. 2002; Andren et al. 2006; Szczęsna et al. 2008). Therefore, monitoring parasite load in wildlife showing negative population trends may be crucial for understanding their status.
The helminth fauna of Eurasian lynx in Europe reported so far consists of 25 species including 11 species of cestodes, one species of trematode, and 14 species of nematodes (Furmaga 1953; Merkusheva and Bobkova 1981; Yushkov 1995; Samuel et al. 2001; Bagrade et al. 2003; Valdmann et al. 2004; Szczęsna et al. 2008; Kornnyushin and Varodi 2010; Deksne et al. 2013; Lavikainen et al. 2013; Haukisalmi et al. 2016). Data from lynx in Poland are limited due to rarity of this species and are based on either scant material or fecal samples, which provide underestimated results of helminth prevalence and intensity (Szczęsna et al. 2008). The presence of parasites such as Diphyllobothrium latum (L., 1758); Hydatigera (=Taenia) rileyi (Loewen, 1929) (misidentification, Taenia sp. (Fagasiński, 1961) according to Abuladze [18]); Spirometra janickii Furmaga, 1953; Alaria alata (Goeze, 1782); Aelurostrongylus abstrusus (Railliet, 1898); Ancylostoma tubaeforme (Zeder, 1800); Eucoleus (=Thominx) aerophilus (Creplin, 1839); Metastrongylus sp.; Nematodirus sp.; Toxocara mystax (=T. cati) (Schrank, 1788) Baylis et Daubney, 1923; and Toxascaris leonina (Linstow, 1902) was confirmed to date (Furmaga 1953; Fagasiński 1961; Okulewicz et al. 2002; Górski et al. 2006; Szczęsna et al. 2006, 2008; Filip and Demiaszkiewicz 2017).
In this study, we present new data based on examination of opportunistically collected lynx carcasses. The aim of the study was to contribute data on the helminth fauna of Eurasian lynx inhabiting Poland based on morphological and molecular analysis of parasites. Due to the protected status of lynx in Poland, such comprehensive analyses give a unique opportunity to increase the knowledge on potential limiting factors for the sustainability of the fragmented population of this large felid.
Material and methods
Animal dissections and collecting of helminths
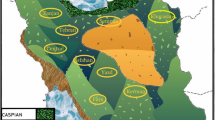
Eleven lynx carcasses were collected in 2007–2017 in northern-eastern and southern Poland (Fig. 1). In all cases, except one which was a road fatality, the lynx were found dead and some of them were strongly emaciated (Table 1). Gastrointestinal tracts and muscle samples were collected for parasitological studies. Isolated helminths were preserved in 70% ethanol for further analysis.
Morphological identification of parasites
For light microscope observation, nematodes were processed in 80% solution of phenol in glycerol, for measurement nematodes were processed in enlightening solution made from lactic acid, phenol, glycerol, and distilled water (1:1:1:1). Cestode scolexes were processed in a 50% solution of glycerol in distilled water. The total amounts of scolexes were kept in Berleses medium, and mature and immature proglottids were stained with iron acetocarmine and mounted in Canada balsam (Ivashkin et al. 1971). Observations were done using Amplival Carl Zeiss Jena and Zeiss Axio Imager M1 microscopes and Leica M165C and MBS-9 stereomicroscopes.
Species identification of parasites was based on morphological features. In the case of damaged specimens, a standard coproscopic parasite eggs identification was additionally performed. Comparison with the collection of helminths from the Department of Parasitology of the Schmalhausen Institute of Zoology, specific keys to parasitic nematodes, Mesocestoidata, and Taeniata was used (e.g., Skryabin et al. 1951, 1952; Mozgovoy 1953; Abuladze 1964; Ryzhikov 1978). Infection of Eurasian lynx with helminths was presented by standard parameters: prevalence—number of infected hosts divided by the total number of dissected animals (shown in the form of simple fraction); mean intensity—the total number of parasites divided by the number of infected lynx; mean abundance—the total number of parasites divided by the total number of analyzed animals (Bush et al. 1997).
Digestion and molecular identification of Trichinella species
Muscle samples (diaphragm or foreleg muscle) from eight lynx (15 g each) were individually examined for the presence of Trichinella muscle larvae using the magnetic stirrer method for sample digestion as described by Kapel and Gamble (2000). Obtained muscle larvae were identified based on morphological characteristics and counted, and the number of larvae per gram of muscle tissue (LPG) was calculated. Larvae were washed several times in distilled water and stored in 96% ethanol until speciation by the multiplex polymerase chain reaction (PCR).
DNA was isolated from a pool of five larvae from each sample (if available). Extraction of DNA and PCR amplification was performed according to Pozio and La Rosa (2003) modified by using two pairs of primers: ESVF (5′-GTTCCATGGAACAGCAGT) and ESVR (5′-CGAAAACATACGACAACTGC) amplifying the expansion segment V (ESV) region in all known Trichinella species and genotypes and TBF (5′-GCTACATCCTTTTGATCGGTT) and TBR (5′-AGACACAATATCAACCACAGTACA) that amplify a partial region of ITS-1 specific for T. britovi Britov et Boev, 1972. For amplification, 5 μl DNA was added to the PCR reaction mix (vol. 25 l) by Eppendorf® and PCR was performed in a XP ThermalCycler (BIOER Technology Co., LTD.). Amplification was carried out under the following cycling conditions: 40 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and elongation at 72 °C for 1 min. The amplification products (5 μl) were separated on a 1.6% standard agarose gel and stained with GoldView™ Nucleic Acid Stain before visualization. All PCRs included negative and positive controls—reference strains T. spiralis Owen, 1835 (ISS 004); T. nativa Britov et Boev, 1972 (ISS 042); T. britovi (ISS 1088); and T. pseudospiralis Garkavi, 1972 (ISS 013).
Genetic identification and phylogenetic analysis of Taenia species
To verify the results of microscope examination of the Taenia species that could be misidentified by morphological features, we applied three primer pairs, constructed by Jia et al. (2010), designed for purposes of comparative mitogenomics of Taenia species. The three primer pairs enabled the amplification of four distinct mtDNA fragments containing cytochrome c oxidase (cox, approx. 470 bp), nicotinamide dehydrogenase subunits 1 (nad1, approx. 460 bp) and 5 (nad5, approx. 600 bp), and the small unit of rRNA (rrnS, approx. 400 bp). Amplifications of the fragments were performed in 10 μl of reaction mixture, including 2 μl of the DNA, 5 μl of PCR HotStarTaq Master Mix Kit, and 1 μl of each of two primers (5 pmol/μl). Thermal cycling conditions: 35 cycles of denaturation at 95 °C for 60 s, annealing at 58 °C for 45 s (T_nad1 and T_nad5), 65 °C for 45 s (T-rrnS), and 54 °C for 60 s (Cox1), and elongation at 72 °C for 10–15 min.
To increase the possibility to detect all possible Taenia species, we sampled 1–4 distinct parasites from each lynx, depending on their individual availability. The obtained sequences were BLASTed using the NCBI online tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) (Altschul et al. 1990). Sequence alignments between the obtained sequences and the evolutionarily closest sequences were performed using ClustalW Multiple Alignment option delivered within BioEdit tools (Hall 1999). Molecular phylogenetic analysis of the lynx parasites by Maximum Likelihood method based on the Tamura-Nei model within MEGA6 tool (Tamura and Nei 1993; Tamura and Kumar 2004) was performed for all the samples and all three mtDNA fragments.
Mean genetic distances between taxa (average evolutionary divergence over sequence pairs between groups of sequences) and within taxa, expressed as the number of base substitutions per site from averaging over all sequence pairs between groups, were estimated based on maximum composite likelihood model (Tamura and Nei 1993) using MEGA6 (Tamura et al. 2013).
Results
All lynx we examined were infected with helminths. We found eight species of parasites, consisting of four species of cestodes: Mesocestoides lineatus, Spirometra sp., Taenia krabbei, and Taenia lynciscapreoli and four species of nematodes: Ancylostoma tubaeforme, Toxocara cati, Toxascaris leonina, and Trichinella britovi (Tables 1 and 2). The four cestode species mentioned above and one nematode (T. britovi) are reported for the first time in lynx inhabiting Poland.
Most prevalent was T. lynciscapreoli, found in 8 out of 11 studied lynx. We found Taenia krabbei in five lynx and both M. lineatus and Spirometra sp. in one of the lynx. Three individuals were infected with Trichinella sp. with intensity of the latter at 0.1 to 3.2 LPG. T. britovi was confirmed by PCR in two lynx. The highest infection intensity was observed for M. lineatus and the lowest for Spirometra sp. (see Table 3). We observed an untypical location of Spirometra sp., in the large intestine. The results of interspecific mtDNA alignment are presented in Fig. 2a–d.
Phylograms for the four mtDNA fragments of the lynx parasite DNA samples collected in the study (in bold type) and the reference sequences from GeneBank (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/). Evolutionary relationships of the taxa were implied using the Neighbor-Joining method (Tamura and Nei 1993) embedded in MEGA6 software (Tamura et al. 2013). The evolutionary distances were computed using the maximum composite likelihood method (Tamura and Kumar 2004) and are expressed in units of the number of base substitutions per site. The molecular phylogenies were drawn based on the following mtDNA fragments: a nicotinamide dehydrogenase subunit 1 (nad1); b nicotinamide dehydrogenase 5 (nad5), c small unit of rRNA (rrnS); and d cytochrome c oxydase (cox). Roman numerals indicate lyx ID, while Arabic numerals preceded by a dash indicate subsequent sampled parasite individuals
Discussion
Our study provided new information on the helminth species of lynx in the territory of Poland. Until now, the accumulated data from the literature along with the results of this study show that the lynx in this area can be a host to at least 14 species of helminths. This data is important considering that lynx populations in Poland are unstable or decreasing (Von Arx et al. 2004), and in central Europe, they are also strongly fragmented and limited to forest areas (Kowalczyk et al. 2015; Schmidt 1998). Therefore, every risk that may contribute to deepening the vulnerability of the population, including parasitic invasions, must be taken into account in conservation strategies.
Lynx feed on ungulates (~ 90% of prey) with roe deer (Capreolus capreolus) and red deer (Cervus elaphus) being the main prey species (Okarma et al. 1997), with smaller animals being preyed upon only occasionally. Intermediate hosts for T. lynciscapreoli and T. krabbei Moniez, 1789, are roe deer and other cervids (Al-Sabi et al. 2013; Haukisalmi et al. 2016). Thus, the relatively high Taenia sp. infection prevalence stated in our study is directly connected with lynx feeding habits. According to our best knowledge, this is the first reported case of lynx as the definitive host for T. krabbei that usually uses canids as its definitive hosts (Priemer et al. 2002; Lavikainen et al. 2011). The life cycle of M. lineatus has not yet been clarified. However, the first intermediate host could be oribatid mites, and the range of the second intermediate hosts is quite wide, including species from different classes of vertebrates (Ryzhikov 1978; Yushkov 1995), which could form part of the lynx diet. T. cati and A. tubaeforme are widespread helminths of cats with A. tubaeforme being a specific parasite of felids. Both species infect hosts percutaneously in dens, during feeding (engulfment of eggs or larvae in rodents or insects or with milk) or through the placental barrier. However, no larvae migrans of these nematodes were found in our study.
Our data suggest that the confirmed southern range of T. lynciscapreoli should be extended. Up to-date, the species has been found and reported in Lohja, Hyvinkää, Hausjärvi (Southern Finland), Mustasaari, Sauvo (Western Finland), Mikhailovskiy raion (Altai Krai), Kolosovsky, and Bolsheukovsky raions (Omskaya oblast, Western Siberia) in the Russian Federation (Haukisalmi et al. 2016).
To our knowledge, we report the first finding of a Trichinella parasite in Eurasian lynx from Poland. T. britovi was detected in two adult females from Białowieża Forest and an adult male from Augustów Forest. The presence of Trichinella in lynx is not surprising, as the species is known to feed occasionally on carnivorous and scavenging mammals (Okarma et al. 1997). Trichinellosis in Eurasian lynx was found in 22 to 67% of individuals in Estonia (Järvis and Miller 1999; Valdmann et al. 2004; Malakauskas et al. 2007; Pozio 2013) in 46 to 98% in Republic of Latvia (Bagrade et al. 2003; Malakauskas et al. 2007) and in Finland, where the prevalence in lynx is the highest among free-living carnivores—up to 70% (Airas et al. 2010). T. britovi was detected also in lynx from Slovakia (Hurníková et al. 2009) and in 27% of Swiss lynx (Frey et al. 2009). This species is also prevalent in other wild mammals in Poland, infecting mainly red fox (Cabaj et al. 2000; Cabaj et al. 2004), but also found in wild boar (Sus scrofa L., 1758) (Bilska-Zając et al. 2013), wolf (Cabaj 2006), martens and badger (Moskwa et al. 2012), and American mink (Neovison vison Schreber, 1777) (Hurníková et al. 2016).
The number of dissected animals in this study was too small to derive conclusions about possible relationships between parasite infestation intensity and lynx mortality or morbidity. However, in the majority of cases, the dead lynx were found emaciated and without an apparent cause of death. However, the harmful influence of helminth invasion on animal health status has been shown previously (Rodriguez and Carbonell 1998).
This study conducted on the rare and unique material of dead lynx from the territory of Poland has yielded unique knowledge of lynx helmith fauna. All these findings may be helpful in explaining parasite transmission patterns in the areas inhabited by lynx. Five new pathogenic agents discovered in endangered Eurasian lynx on the Polish territory could have a negative impact on its condition and fitness.
References
Abuladze KI (1964) Taeniata—the tapeworms of animals and humans and diseases caused by them. Foundations of Cestodology. Nauka, Moscow, p 540 [In Russian]
Airas N, Saari S, Mikkonen T, Virtala AM, Pellikka J, Oksanen A, Isomursu M, Kilpelä SS, Lim CW, Sukura A (2010) Sylvatic Trichinella spp. infection in Finland. J Parasitol 96:67–76
Al-Sabi MN, Chriél M, Holm E, Jensen TK, Ståhl M, Enemark HL (2013) Reappearance of Taenia ovis krabbei muscle cysts in a roe deer (Capreolus capreolus) in Denmark after 60+ years. Vet Parasitol 196:225–229
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andren H, Linnell JDC, Liberg O, Andersen R, Danell A, Karlsson J et al (2006) Survival rates and causes of mortality in Eurasian lynx (Lynx lynx) in multi-use landscapes. Biol Conserv 131:23–32
Bagrade G, Vismanis K, Kirjušina M, Ozoliņš J (2003) Preliminary results on the helminthofauna of the Eurasian lynx (Lynx lynx) in Latvia. Acta Zool Lituan 13:3–7
Bilska-Zając E, Różycki M, Chmurzyńska E, Marucci G, Cencek T, Karamon J et al (2013) Trichinella species circulating in wild boar (Sus scrofa) populations in Poland. Int J Parasitol 2:211–213
Breitenmoser U, Breitenmoser-Würsten Ch, Okarma T, Kaphegyi T, Kaphegyi-Wallmann U, Müller UM (2000) Action plan for the conservation of the Eurasian lynx (Lynx lynx) in Europe. Council and Europe Publishing, Nature and Environment 112:1–69
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Cabaj W (2006) Wild and domestic animals as permanent Trichinella reservoir in Poland. Wiad Parazytol 52:175–179
Cabaj W, Pozio E, Moskwa B, Malczewski A (2000) Trichinella spiralis and T. britovi in red foxes (Vulpes vulpes) in Poland. Acta Parasitol 45:340–344
Cabaj W, Moskwa B, Pastusiak K, Malczewski A (2004) Trichinosis in free-living animals and pigs in Poland. Med Weter 60:80–83 [In Polish]
Chapron G, Kaczensky P, Linnell JD, von Arx M, Huber D, Andren H et al (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346:1517–1519
Deksne G, Laakkonen J, Näreaho A, Jokelainen P, Holmala K, Kojola I, Sukura A (2013) Endoparasites of the Eurasian lynx (Lynx lynx) in Finland. J Parasitol 99:229–234
Fagasiński A (1961) A contribution to the knowledge of helminth fauna of the lynx and wildcat in Poland. Acta Parasitol Polonica 9:1–6
Filip K, Demiaszkiewicz AW (2017) Endoparasites of Eurasian lynx (Lynx lynx) (Linnaeus, 1758) from enclosure of of Western Pomeranian Nature Society in Jablonowo. Ann Parasitol 63:33–36
Frey CF, Schnuppers ME, Müller N, Ryser-Degiorgis MP, Gottstein B (2009) Assessment of the prevalence of Trichinella spp. in red foxes and Eurasian lynx from Switzerland. Vet Parasitol 159:295–299
Furmaga S (1953) Spirometra janickii sp. n. (Diphyllobothriidae). Acta Parasitol Polonica 1:29–64
Górski P, Zalewski A, Łakomy M (2006) Parasites of carnivorous mammals in Białowieża Primeval Forest. Wiad Parazytol 52:49–53
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Series 41:95–98
Haukisalmi V, Konyaev S, Lavikainen A, Isomursu M, Nakao M (2016) Description and life-cycle of Taenia lynciscapreoli sp. n. (Cestoda, Cyclophyllidea). Zookeys 584:1–23
Hurníková Z, Kołodziej-Sobocińska M, Dvorožňáková E, Niemczynowicz A, Zalewski A (2016) An invasive species as an additional parasite reservoir: Trichinella in introduced American mink (Neovison vison). Vet Parasitol 231:106–109
Hurníková Z, Miterpáková M, Chovancová B (2009) The important zoonoses in the protected areas of the Tatra National Park (TANAP). Wiad Parazytol 55:395–398
Ivashkin VM, Kontrimavichus VL, Nazarova NS (1971) Methods of collecting and studying of helminths of terrestrial mammals. Nauka, Moscow [In Russian]
Järvis T, Miller I (1999) Epizootology of trichinellosis. Eesti Arst 5:414–417 [In Estonian]
Jędrzejewski W, Nowak S, Schmidt K, Jędrzejewska B (2002) The wolf and the lynx in Poland—results of a census conducted in 2001. Kosmos 51:491–499 [In Polish]
Jia WZ, Yan HB, Guo AJ, Zhu XQ, Wang YC, Shi WG, Chen HT, Zhan F, Zhang SH, Fu BQ, Littlewood DTJ, Cai XP (2010) Complete mitochondrial genomes of Taenia multiceps, T. hydatigena and T. pisiformis: additional molecular markers for a tapeworm genus of human and animal health significance. BMC Genomics 11:447
Kapel CMO, Gamble HR (2000) Infectivity, persistence, and antibody response to domestic and sylvatic Trichinella spp. in experimentally infected pigs. Int J Parasitol 30:215–221
Kornnyushin VV, Varodi EI (2010) Helminths of domestic and wild cats in Ukraine. Scientific Journal of the National University of Bioresearch and Management of Natural Resources of Ukraine, Veterinary Medicine, Quality and Safety of Animal Produce 51:113–118 [In Ukrainian]
Kowalczyk R, Górny M, Schmidt K (2015) Edge effect and influence of economic growth on Eurasian lynx mortality in the Białowieża Primeval Forest, Poland. Mammal Res 60:3–8
Lavikainen A, Haukisalmi V, Deksne G, Holmala K, Lejeune M, Isomursu M et al (2013) Molecular identification of Taenia spp. in the Eurasian lynx (Lynx lynx) from Finland. Parasitology 140:653–662
Lavikainen A, Laaksonen S, Beckmen K, Oksanen A, Isomursu M, Meri S (2011) Molecular identification of Taenia spp. in wolves (Canis lupus), brown bears (Ursus arctos) and cervids from North Europe and Alaska. Parasitol Int 60:289–295
Malakauskas A, Paulauskas V, Järvis T, Keidans P, Eddi C, Kapel CMO (2007) Molecular epidemiology of Trichinella spp. in three Baltic countries: Lithuania, Latvia, and Estonia. Parasitol Res 100:687–693
Merkusheva IV, Bobkova AF (1981) Helminths of domestic and wild live animals of Belorussia: catalogue. Nauka i technika, Minsk [In Russian]
Moskwa B, Goździk K, Bień J, Bogdaszewski M, Cabaj W (2012) Molecular identification of Trichinella britovi in martens (Martes martes) and badgers (Meles meles); new host records in Poland. Acta Parasitol 57:402–405
Mozgovoy AA (1953) Ascaridata of animals and human and caused diseases by them. In: Skryabin KI (ed) Foundations of nematology. Nauka, Moscow, p 618 [In Russian]
Okarma H, Jędrzejewski W, Schmidt K, Kowalczyk R, Jędrzejewska B (1997) Predation of Eurasian lynx on roe deer and red deer in Białowieża Primeval Forest, Poland. Acta Theriol 42:203–224
Okulewicz A, Lonc E, Borgsteede FHM (2002) Ascarid nematodes in domestic and wild terrestrial mammals. Polish J Vet Sci 5:277–281
Podgórski T, Schmidt K, Kowalczyk R, Gulczyńska A (2008) Microhabitat selection by Eurasian lynx and its implications for species conservation. Acta Theriol 53:97–110
Pozio E (2013) The opportunistic nature of Trichinella—exploitation of new geographies and habitats. Vet Parasitol 194:128–132
Pozio E, La Rosa G (2003) PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol Biol 216:299–309
Priemer J, Krone O, Schuster R (2002) Taenia krabbei (Cestoda: Cyclophyllidea) in Germany and its delimitation from T. ovis. Zool Anz 241:333–337
Rodriguez A, Carbonell E (1998) Gastrointestinal parasites of the Iberian lynx and other wild carnivores from central Spain. Acta Parasitol 43:128–136
Ryzhikov KM (1978) Tetrabothriata and Mesocestoidata—tapeworms of birds and mammals. Foundations of Cestodology. Nauka, Moscow, p 231 [In Russian]
Samuel WM, Kocan AA, Pybus MJ (2001) Parasitic diseases of wild mammals, 2nd edn. Manson publishing, London
Schmidt K (1998) Maternal behaviour and juvenile dispersal in the Eurasian lynx. Acta Theriol 43:391–408
Schmidt-Posthaus H, Breitenmoser-Würsten C, Posthaus H, Bacciarini L, Breitenmoser U (2002) Causes of mortality in reintroduced Eurasian lynx in Switzerland. J Wildl Dis 40:356–360
Skryabin KI, Shihobalova NP, Schulz RS, Popova TI, Boev SN, Delamure SL (1952) Strongylata. In: Skryabin KI (ed) Keys to parasitic nematodes. Academy of Sciences of USSR, Moscow, p 892 [In Russian]
Skryabin KI, Shihobalova NP, Mozgovoy AA (1951) Oxyurata and Ascaridata. In: Skryabin KI (ed) Keys to parasitic nematodes. Academy of Sciences of USSR, Moscow, p 634 [In Russian]
Szczęsna J, Popiołek M, Schmidt K, Kowalczyk R (2006) The first record of Aelurostrongylus abstrusus (Angistrongylidae: Nematoda) in Eurasian lynx (Lynx lynx L.) from Poland based on fecal analysis. Wiad Parazytol 52:321–322
Szczęsna J, Popiołek M, Schmidt K, Kowalczyk R (2008) Coprological study on helminth fauna in Eurasian lynx (Lynx lynx) from the Białowieza Primeval Forest in eastern Poland. J Parasitol 94:981–984
Tamura K, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Nat Acad Sci USA 101:11030–11035
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Valdmann H, Moks E, Talvik H (2004) Helminth fauna of Eurasian lynx (Lynx lynx) in Estonia. J Wildl Dis 40:356–360
Von Arx M, Breitenmoser-Würsten C, Zimmermann F, Breitenmoser U (2004) Status and conservation of the Eurasian lynx (Lynx lynx) in 2001. KORA Bericht 19
Yushkov VF (1995) Helminths of mammals. Fauna of european north-east of Russia. Nauka, Saint-Petersburg, p 201 [In Russian]
Acknowledgements
We would like to thank Amy Eycott and Jamie Cottam and for correcting the English of the manuscript.
Material was deposited in the scientific collection of the Mammal Research Institute, Polish Academy of Sciences in Białowieża. For species identification, material has been made available temporarily to the Department of Parasitology of the Schmalhausen Institute of Zoology NAS of Ukraine.
Funding
The study was supported by the European Commission’s 7th Framework Programme, Marie Curie Actions project “Biodiversity of East European and Siberian large mammals on the level of genetic variation of populations” (PIRSES-GA-2009-247652-BIOGEAST) conducted by the Mammal Research Institute Polish Academy of Sciences in Białowieża, Poland, in cooperation with Schmalhausen Institute of Zoology in Kyiv, Ukraine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Section Editor: Herbert Auer
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kołodziej-Sobocińska, M., Yakovlev, Y., Schmidt, K. et al. Update of the helminth fauna in Eurasian lynx (Lynx lynx) in Poland. Parasitol Res 117, 2613–2621 (2018). https://doi.org/10.1007/s00436-018-5953-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5953-0