Abstract
Sharpey’s fibres are known mainly as providing anchorage between tooth and the periodontal ligament but they occur also in other types of bones. In the postcranial skeleton these fibres are usually present at the muscle or tendon attachment sites. They were reported in all major groups of extant vertebrates, as well as in putative lissamphibian ancestors—temnospondyls and lepospondyls. However, it was recently stated that their presence was very rarely described in extant amphibians. In limbs, they were reported predominantly from proximal bones. They have not yet been reported from phalanges, which are the most commonly sectioned amphibian bones. Here, we describe phalangeal histology of nine species representing most major clades of lissamphibians. These results show that Sharpey’s fibres occur commonly in lissamphibian phalanges. In shaft, they are radially oriented and occur in the periosteal bone, at sites of tendon attachment. They can also occur in the metaphysis and contact the cartilage. This may provide a basis for foot muscle reconstructions in fossil amphibians.
Similar content being viewed by others
Introduction
Sharpey’s fibres (SF) are poorly mineralised fibres of the connective tissue, composed mostly of several types of collagen, elastin or tenascin (e.g. Francillon-Vieillot et al. 1990; Aaron 2012). They are known primarily as providing attachment between tooth and a periodontal ligament (e.g. Ho et al. 2007). However, they occur also in other body parts, where they most commonly attach muscles, ligaments or tendons to collagen fibres present in the periosteal bone (Francillon-Vieillot et al. 1990; Aaron 2012) but can also be present in osteoderms, in which they probably serve as anchorage to the skin (e.g. Witzmann and Soler-Gijón 2010). Some authors even restrict the definition of SF only to collagen fibres inserting the bone (Simmons et al. 1993; after Hall 2015) but it seems that this definition is less commonly used than the broader one (Aaron 2012; Hall 2015). Although the presence of the periosteal SF is less well documented than those involved in tooth anchorage, they have been described in most of the major groups of extant tetrapods, such as mammals (Singh et al. 1974; Aaron 2012; Warshaw et al. 2017), birds (Genbrugge et al. 2012; Petermann and Sander 2013) and reptiles (Castanet et al. 1988; Hutchinson 2002; Suzuki et al. 2002, 2003). Recently, Clemente-Carvalho et al. (2009) described SF from the skull of brachycephalid frogs and stated that this is only the second reference to SF in amphibians, besides the report of their occurrence in the femur of the American bullfrog Lithobates catesbeianus (Felisbino and Carvalho 2000). However, these fibres have also been described in the osteoderms of some frogs (Ruibal and Shoemaker 1984; Quinzio and Fabrezi 2012), the stylopodial bones of the conrauid frog Conraua goliath and the cryptobranchiid salamander Andrias davidianus (Canoville et al. 2018), the skull of an extinct cryptobranchiid Eoscapherpeton asiaticum (Skutschas and Boitsova 2017) and in the vertebrae of an extant caecilian Ichthyophis kohtaoensis (Castanet et al. 2003). Interestingly, SF have been more commonly described in fossil stem-amphibians than in living ones, including both dermal and endochondral bones of both temnospondyls and lepospondyls (e.g. Witzmann 2009; Konietzko-Meier and Sander 2013; Danto et al. 2016)—two groups which may be ancestral to lissamphibians, a clade including all living amphibians (Marjanović and Laurin 2013).
Studying phalanges is less invasive than using more proximal bones, which requires sacrificing the life of an individual, and thus is preferred particularly in endangered species, such as most lissamphibians. Thus, phalanges are the bones most commonly used in the histological age determination of amphibians (i.e. skeletochronology; Sinsch 2015). Despite the fact that phalanges were used in skeletochronological studies on wide range of extant amphibians, to our knowledge the presence of SF has not yet been reported in those bones. The aim of our study was to find out whether SF occur commonly or rarely in lissamphibian phalanges. This could also provide an experimental basis for inferring muscle attachment sites in fossil amphibians. Numerous muscles attach to the amphibian foot, including several attaching to the fourth digit, responsible for its flexion and extension. Most of them have tendinous attachment sites (entheses), where SF are usually particularly abundant.
For this purpose we investigated bone histology of nine species which belong to several major clades of extant amphibians (Lissamphibia) and represent different modes of life and locomotion (Table 1)—factors which may affect bone histology (e.g. Laurin et al. 2004; Canoville and Laurin 2009).
Materials and methods
Taxon and specimen sampling
The sample consisted of adult individuals of Bombina bombina, Bufo bufo, Bufotes viridis, Hyla arborea, Pelobates fuscus, Pelophylax ridibundus, Rana temporaria, Salamandra salamandra and Lissotriton vulgaris (see Fig. 1 for their phylogenetic relationships). Each of these species was represented by three specimens (with the exception of H. arborea, of which two specimens were available). All of them come from the collections of the Department of Evolutionary Biology and Conservation of Vertebrates, University of Wrocław and the Institute of Zoology, Poznań University of Life Sciences. No animal was killed for the purpose of this study.
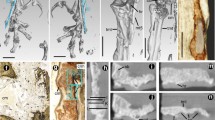
Simplified dendrogram showing relationships of species used in this study (after Frost et al. 2006; Pyron and Wiens 2011). It shows also the occurrence of Sharpey’s fibres in the phalanges of the fourth toe of the hindlimb. M metaphysis, D diaphysis (M1 metaphysis of the first phalanx, D2 diaphysis of the second phalanx and so on). “+” observed, “−” not observed
Specimen preparation and analysis
The fourth digit of the right hind limb was taken (in contrary to other digits, it has four phalanges). It was manually cleaned of associated soft tissues. The phalanges were decalcified in a 1:1 mixture of 10% formic acid and 4% formalin for 1–4 h, depending on the size of the bone. To remove traces of decalcifying agent, bones were washed in four changes of distilled water, 15 min each, and stored in 70% ethanol. We embedded the bones in a tissue freezing medium (Leica, Biosystems Nussloch GmbH, Germany) and sectioned into 10 µm thick sections on a freezing microtome (Leica CM 1850 UV), and stained with 0.05% cresyl violet, following Rozenblut and Ogielska (2005). In all cases phalanges were sectioned from proximal to distal epiphysis.
We tested also several other methods of sectioning and staining, utilised commonly in histological studies, using third phalanx of B. bufo as an example. All phalanges were dehydrated, cleared in xylene, then embedded into paraffin, sectioned using Leica RM2255 microtome into 7 µm thick sections and mounted on slides. Several different methods of staining were tested: (1) Mallory’s solution, in which cartilage and bone stain red and collagen fibres—deep blue; (2) Mallory’s trichrome, in which cartilage and bone matrix stain blue and collagen fibres—dark blue (3) and Delafield’s haematoxylin and eosin, in which collagen fibres stain pink due to the acid aniline eosin. However, these methods performed more poorly than the one utilising freezing microtome and staining with cresyl violet. Additionally, unstained sections were observed under polarised light and by dark-field microscopy. The sections were analysed under a light microscope (Carl Zeiss Axioskop 20) at different magnifications (ranging from 20× to 40×).
We used Burton (2004) as a model for amphibian pes musculature.
Results
Bone histology
The compacta was the thickest in the middle part of the diaphysis and the medullary cavity expanded toward the epiphyses. The bone tissue was parallel-fibred in most cases but in some thin-sections of salamander bones we observed a lamellar bone. Lines of arrested growth (LAGs) which reflect cessation of bone growth during hibernations were well visible and their number confirmed that all studied individuals were adult (actively growing animals may differ in the histological structure of the bone; Castanet et al. 2003; Canoville and Laurin 2009). The bones were rather poorly vascularised (e.g. B. viridis in Fig. 2) or avascular. In the periosteal bone of S. salamandra, L. vulgaris and P. fuscus there was a single, large nutrient canal, in B. bombina we observed two nutrient canals on the opposite parts of the periosteal bone, while neobatrachians (ranids, bufonids and Hyla) tended to show larger number of nutrient canals. The degree of resorption of the periosteal bone and apposition of endosteal bone determined the size of the medullary cavity in the mid-diaphysis. The largest cavities were observed in B. bufo, B. viridis and H. arborea, while other species had much narrower cavities. In bufonids, the margin of the medullary cavity was irregularly resorbed, creating erosion bays. In H. arborea there was a balance between bone resorption and apposition, resulting in nearly oval and smooth borders of the cavity. In B. bufo, B. viridis and H. arborea the endosteal bone formed only a thin ring around the medullary cavity. The other species had better developed endosteal bone that filled large parts of the resorbed cavity. The smallest degree of resorption was observed in S. salamandra, L. vulgaris and B. bombina.
Sharpey’s fibres
Distinct bundles of fibres occurred in phalanges of all sampled species, most commonly in the II and III phalanges. However, they were not observed in all phalanges of every individual (Fig. 1). In the diaphysis, the fibres were radially oriented, running perpendicularly or slightly obliquely to the bone. They were most numerous in the bony ridges, where tendons attach, and where the periosteal SF occur most commonly (morphologically, they are most similar to type 1 SF in Konietzko-Meier and Sander 2013). They were well visible under dark-field and strongly birefringent under polarised light in B. bufo (Fig. 3) but the birefringence was more poorly characterised in S. salamandra. In the proximal phalanx of anurans, they were particularly well developed on the ventral side of the metaphysis, where medial M. lumbricalis brevis had tendinous attachment. In urodeles, SF were present also on the dorsal side of the bone, at sites where M. extensoris brevis superficialis and M. extensor brevis profundus attached indirectly to the bone. In the second phalanx they also occurred on the ventral side of the metaphysis, where M. lumbricalis longus, lateral M. lumbricalis brevis, and M. interphalangealis proximalis had their tendinous attachment sites. In urodeles, SF also occurred on the dorsal surface, where lateral M. extensoris brevis superficialis attached to the phalanx through the tendon. In the third phalanx, we did not observe SF on the dorsal side in anurans but they were present in the urodeles. On the ventral side, the fibres were present in all examined species, in both shaft and metaphysis, where M. interphalangealis distalis and medial M. lumbricalis brevis have entheses. In the distal phalanx, SF are present on ventral and dorsal surfaces in both anurans and salamanders. To this bone, M. extensor brevis distalis and tendo superficialis are attached. SF may contact the medullary cavity or the endosteal bone (if present) but we did not observe them penetrating the endosteal bone (Fig. 4). SF may also be present in the metaphysis, where they can contact the hyaline cartilage (Fig. 5). They penetrated the bone beneath the epiphysis and between metaphysis and lateral articular surface, and are present on both dorsal and ventral sides. The best developed SF were present in two toad species, while in Bombina they were only faintly visible. In the places where SF occurred, growth marks were difficult to observe.
Sharpey’s fibres in transverse sections of the third phalanx (diaphysis) of: a Bombina bombina, b Bufo bufo, c Bufotes viridis, d Hyla arborea, e Pelobates fuscus, f Pelophylax ridibundus, g Rana temporaria, h Salamandra salamandra, i Lissotriton vulgaris. White arrows—Sharpey’s fibres, t tendon. Scale bar equals 100 µm. Dorsal side upwards
Sharpey’s fibres in different phalanges: a second phalanx (diaphysis) of B. bufo with SF located on the left side, b fourth phalanx (diaphysis) of H. arborea with SF located both on the ventral and the dorsal side, c first phalanx (diaphysis) of S. salamandra with SF located on the ventral side, d metaphysis of the third phalanx of R. temporaria with SF located on the lateral side. White arrows—Sharpey’s fibres, et enthesis. Scale bar equals 100 µm. Dorsal side upwards
Discussion
The intraspecific differences in bone tissue type observed in some species are not surprising. The histological bone structure may be affected by many factors, both internal and external, such as individual age, sex and environmental or geographic aspects (e.g. Alcobendas and Castanet 2000; Castanet et al. 2003; Kaczmarski et al. 2016).
Presence of SF is well documented in many types of bones (both cranial and postcranial, dermal and endochondral) of Palaeozoic and Mesozoic amphibians, including both temnospondyls and lepospondyls (e.g. Witzmann 2009; Mukherjee et al. 2010; Sanchez et al. 2010; Konietzko-Meier and Klein 2013; Konietzko-Meier and Sander 2013; Canoville and Chinsamy 2015; Danto et al. 2016; Gruntmejer et al. 2016). At least one of these two groups gave rise to lissamphibians (see review in Marjanović and Laurin 2013). However, histological studies on fossil lissamphibians are rare and SF have not yet been reported in extinct caudates (bone histology of fossil salientians is even more poorly studied, so no comparison can be made), except in the frontal of the Late Cretaceous cryptobranchiid Eoscapherpeton (de Buffrénil et al. 2015; Skutschas and Stein 2015; Skutschas and Boitsova 2017). The discovery that SF are common in extant amphibians strongly suggests that their presence in extinct amphibians of similar mode of life is expected (at least in phalanges). Recently, Petermann and Sander (2013) presented an experimental basis for inferring muscle attachment sites based on histological sections of amniote femora. Our study provides an extension to Lissamphibia and may serve as a basis for future studies investigating different parts of the skeleton.
Clemente-Carvalho et al. (2009) claimed that before their publication SF were mentioned only in Felisbino and Carvalho (2000), but these fibres are clearly visible in figures provided by some other authors (e.g. Guarino et al. 2011; Sinsch 2015). However, they were unlabelled, even in those in which other histological structures have been described (e.g. Sinsch 2015).
In two species of toads and in Salamandra, which are among the most terrestrial of lissamphibians, SF were developed more prominently (i.e. the fibres were thicker and covered greater area of the section) than in species spending more time in water, such as the fire-bellied toads (compare Fig. 4b, c, g with a). Thus, one could suspect that this may be related to greater forces acting on the limbs during terrestrial locomotion. However, SF are very well developed in femora and humeri of the aquatic Chinese salamander Andrias davidianus (Canoville et al. 2018), as well as in the Triassic temnospondyl Metoposaurus krasiejowensis, which is interpreted as almost exclusively aquatic (nonetheless, this amphibian was probably able to burrow—this requires strong muscles, which would be consistent with the presence of well developed SF; Konietzko-Meier and Sander 2013). On the other hand, we did not observe well developed SF in P. fuscus, despite partially burrowing lifestyle of this amphibian. However, in closely related P. varaldii these fibres can readily be observed in at least some specimens (Guarino et al. 2011). Also, it should be noted that the presence of SF may be dependent on a number of physiological stimuli, at least in mammals. These include influence of hormones (such as estrogen), degree of physical activity, ageing or pathologies such as osteoporosis or osteoarthritis (Aaron 2012). Explaining the reasons of these differences in amphibians requires further studies.
The fact that SF may obscure some of the growth marks, such as LAGs, potentially hinders skeletochronological studies, especially when only incomplete bones are available (e.g. Castanet et al. 1988). Thus, for skeletochronological analyses it may be advisable to use—if possible—the proximal rather than ultimate or penultimate phalanges, as these phalanges have relatively weakly developed SF.
References
Aaron JE (2012) Periosteal Sharpey’s fibers: a novel bone matrix regulatory system? Front Endocrinol 3:98. https://doi.org/10.3389/fendo.2012.00098
Alcobendas A, Castanet J (2000) Bone growth plasticity among populations of Salamandra salamandra: interactions between internal and external factors. Herpetologica 56:14–25
Burton TC (2004) Muscles of the pes of hylid frogs. J Morphol 260:209–233. https://doi.org/10.1002/jmor.10204
Canoville A, Chinsamy A (2015) Bone microstructure of the stereospondyl Lydekkerina huxleyi reveals adaptive strategies to the harsh post Permian-extinction environment. Anat Rec 298:1237–1254. https://doi.org/10.1002/ar.23160
Canoville A, Laurin M (2009) Microanatomical diversity of the humerus and lifestyle in lissamphibians. Acta Zool 90:110–122. https://doi.org/10.1111/j.1463-6395.2008.00328.x
Canoville A, Laurin M, de Buffrénil V (2018) Quantitative data on bone vascular supply in lissamphibians: comparative and phylogenetic aspects. Zool J Linn Soc 182:107–128. https://doi.org/10.1093/zoolinnean/zlx016
Castanet J, Newman DG, Saint Girons H (1988) Skeletochronological data on the growth, age, and population structure of the tuatara, Sphenodon punctatus, on Stephens and Lady Alice Islands, New Zealand. Herpetologica 44:25–37
Castanet J, Francillon-Vieillot H, de Ricqlès A, Zylberberg L (2003) The skeletal histology of the Amphibia. In: Heatwole H, Davies M (eds) Amphibian biology, vol 5. Surrey Beatty and Sons, Chipping Norton, pp 1598–1683
Clemente-Carvalho RBG, Antoniazzi MM, Jared C, Haddad CFB, Alves ACR, Rocha HS, Pereira GR, Oliveira DF, Lopes RT, dos Reis SF (2009) Hyperossification in miniaturized toadlets of the genus Brachycephalus (Amphibia: Anura: Brachycephalidae): microscopic structure and macroscopic patterns of variation. J Morphol 270:1285–1295. https://doi.org/10.1002/jmor.10755
Danto M, Witzmann F, Fröbisch NB (2016) Vertebral development in Paleozoic and Mesozoic tetrapods revealed by paleohistological data. PLoS One 11:e0152586. https://doi.org/10.1371/journal.pone.0152586
de Buffrénil V, Canoville A, Evans SE, Laurin M (2015) Histological study of karaurids, the oldest known (stem) urodeles. Hist Biol 27:109–114. https://doi.org/10.1080/08912963.2013.869800
Felisbino SL, Carvalho HF (2000) The osteochondral ligament: a fibrous attachment between bone and articular cartilage in Rana catesbeiana. Tissue Cell 32:527–536. https://doi.org/10.1054/tice.2000.0143
Francillon-Vieillot H, de Buffrénil V, Castanet J, Géraudie J, Meunier FJ, Sire JY, Zylberberg L, de Ricqlès A (1990) Microstructure and mineralization of vertebrate skeletal tissues. In: Carter JG (ed) Skeletal biomineralization: patterns, processes and evolutionary trends. Van Nostrand Reinhold, New York, pp 471–530. https://doi.org/10.1029/SC005p0175
Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, De Sá RO, Channing A, Wilkinson M, Donnellan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC (2006) The amphibian tree of life. Bull Am Mus Nat Hist 297:1–370. https://doi.org/10.1206/0003-0090(2006)297[0001:TATOL]2.0.CO;2
Genbrugge A, Adriaens D, De Kegel G, Brabant L, Van Hoorebeke L, Podos J, Dirckx J, Aerts P, Herrel A (2012) Structural tissue organization in the beak of Java and Darwin’s finches. J Anat 221:383–393. https://doi.org/10.1111/j.1469-7580.2012.01561.x
Gruntmejer K, Konietzko-Meier D, Bodzioch A (2016) Cranial bone histology of Metoposaurus krasiejowensis (Amphibia, Temnospondyli) from the Late Triassic of Poland. PeerJ 4:e2685. https://doi.org/10.7717/peerj.2685
Guarino FM, de Pous P, Crottini A, Mezzasalma M, Andreone F (2011) Age structure and growth in a population of Pelobates varaldii (Anura, Pelobatidae) from northwestern Morocco. Amphib Reptil 32:550–556. https://doi.org/10.1163/017353711X605678
Hall BK (2015) Bones and cartilage, 2nd edn. Academic, London. https://doi.org/10.1016/B978-0-12-416678-3.00048-3
Ho SP, Marshall SJ, Ryder MI, Marshall GW (2007) The tooth attachment mechanism defined by structure, chemical composition and mechanical properties of collagen fibers in the periodontium. Biomaterials 28:5238–5245. https://doi.org/10.1016/j.biomaterials.2007.08.031
Hutchinson JR (2002) The evolution of hindlimb tendons and muscles on the line to crown-group birds. Comp Biochem Physiol A 133:1051–1086. https://doi.org/10.1016/S1095-6433(02)00158-7
Kaczmarski M, Kolenda K, Rozenblut-Kościsty B, Sośnicka W (2016) Phalangeal bone anomalies in the European common toad Bufo bufo from polluted environments. Environ Sci Pollut Res 23:21940–21946. https://doi.org/10.1007/s11356-016-7297-6
Konietzko-Meier D, Klein N (2013) Unique growth pattern of Metoposaurus diagnosticus krasiejowensis (Amphibia, Temnospondyli) from the Upper Triassic of Krasiejów. Palaeogeogr Palaeoclimatol Palaeoecol 370:145–157. https://doi.org/10.1016/j.palaeo.2012.12.003
Konietzko-Meier D, Sander PM (2013) Long bone histology of Metoposaurus diagnosticus (Temnospondyli) from the Late Triassic of Krasiejów (Poland) and its paleobiological implications. J Vertebr Paleontol 33:1003–1018. https://doi.org/10.1080/02724634.2013.765886
Laurin M, Girondot M, Loth M-M (2004) The evolution of long bone microstructure and lifestyle in lissamphibians. Paleobiology 30:589–613. https://doi.org/10.1666/0094-8373(2004)030<0589:TEOLBM>2.0.CO;2
Marjanović D, Laurin M (2013) The origin(s) of extant amphibians: a review with emphasis on the “lepospondyl hypothesis”. Geodiversitas 35:207–272. https://doi.org/10.5252/g2013n1a8
Mukherjee D, Ray S, Sengupta DP (2010) Preliminary observations on the bone microstructure, growth patterns, and life habits of some Triassic temnospondyls from India. J Vertebr Paleontol 30:78–93. https://doi.org/10.1080/02724630903409121
Petermann H, Sander M (2013) Histological evidence for muscle insertion in extant amniote femora: implications for muscle reconstruction in fossils. J Anat 222:419–436. https://doi.org/10.1111/joa.12028
Pyron RA, Wiens JJ (2011) A large-scale phylogeny of Amphibia, including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phylogenet Evol 61:543–583. https://doi.org/10.1016/j.ympev.2011.06.012
Quinzio S, Fabrezi M (2012) Ontogenetic and structural variation of mineralizations and ossifications in the integument within ceratophryid frogs (Anura, Ceratophryidae). Anat Rec 295:2089–2103. https://doi.org/10.1002/ar.22601
Rozenblut B, Ogielska M (2005) Development and growth of long bones in European water frogs (Amphibia: Anura: Ranidae), with remarks on age determination. J Morphol 265:304–317. https://doi.org/10.1002/jmor.10344
Ruibal R, Shoemaker V (1984) Osteoderms in amphibians. J Herpetol 18:313–328. https://doi.org/10.2307/1564085
Sanchez S, Germain D, de Ricqlès A, Abourachid A, Goussard F, Tafforeau P (2010) Limb-bone histology of temnospondyls: implications for understanding the diversification of palaeoecologies and patterns of locomotion of Permo-Triassic tetrapods. J Evol Biol 23:2076–2090. https://doi.org/10.1111/j.1420-9101.2010.02081.x
Simmons DJ, Menton DN, Miller S, Lozano R (1993) Periosteal attachment fibers in the rat calvarium. Calcif Tissue Int 53:424–427
Singh IJ, Tonna EA, Gandel CP (1974) A comparative histological study of mammalian bone. J Morphol 144:421–437. https://doi.org/10.1002/jmor.1051440404
Sinsch U (2015) Review: Skeletochronological assessment of demographic life-history traits in amphibians. Herpetol J 25:5–13
Skutschas P, Boitsova EA (2017) Histology of sculptured cranial dermal bones of of the stem salamander Kokartus honorarius (Amphibia: Caudata) from the Middle Jurassic of Kyrgyzstan. Hist Biol 29:423–429. https://doi.org/10.1080/08912963.2016.1171859
Skutschas P, Stein K (2015) Long bone histology of the stem salamander Kokartus honorarius (Amphibia: Caudata) from the Middle Jurassic of Kyrgyzstan. J Anat 226:334–347. https://doi.org/10.1111/joa.12281
Suzuki D, Murakami G, Minoura N (2002) Histology of the bone-tendon interfaces of limb muscles in lizards. Ann Anat 184:363–367. https://doi.org/10.1016/S0940-9602(02)80057-7
Suzuki D, Murakami G, Minoura N (2003) Crocodilian bone-tendon and bone-ligament interfaces. Ann Anat 185:425–433. https://doi.org/10.1016/S0940-9602(03)80100-0
Warshaw J, Bromage TG, Terranova CJ, Enlow DH (2017) Collagen fiber orientation in primate long bones. Anat Rec 300:1189–1207. https://doi.org/10.1002/ar.23571
Witzmann F (2009) Comparative histology of sculptured dermal bones in basal tetrapods, and the implications for the soft tissue dermis. Palaeodiversity 2:233–270
Witzmann F, Soler-Gijón R (2010) The bone histology of osteoderms in temnospondyl amphibians and in the chroniosuchian Bystrowiella. Acta Zool 91:96–114. https://doi.org/10.1111/j.1463-6395.2008.00385.x
Acknowledgements
We thank Mikołaj Kaczmarski (Poznań University of Life Sciences) for providing specimens of the spadefoot toad. Aleksandra Puchtel, Karolina Musiał and Anna Półtorak (University of Wrocław) helped with preparation of some specimens. We thank Maria Ogielska and Bartosz Borczyk (University of Wrocław) for support, criticism and helpful discussions. Detailed comments made by three anonymous referees on an earlier version of the manuscript greatly improved the article.
Funding
This study was funded by Polish Ministry of Science and Higher Education (Grant numbers 0420/1408/16, 0420/1409/16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This study did not use live animals and thus did not require approval of bioethical commission.
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kolenda, K., Najbar, A., Rozenblut-Kościsty, B. et al. Common occurrence of Sharpey’s fibres in amphibian phalanges. Zoomorphology 137, 329–336 (2018). https://doi.org/10.1007/s00435-018-0400-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-018-0400-4