Abstract
Purpose
Clinical practice guidelines provide recommendations for the management of diseases. In orphan conditions such as uveal melanoma (UM), guideline developers are challenged to provide practical and useful guidance even in the absence of high-quality evidence. Here, we assessed the methodological quality and identified deficiencies of international guidelines on UM as a base for future guideline development.
Methods
A systematic search was carried out in guideline databases, Medline and Embase until 27th May 2019 for guidelines on UM published between 2004 and 2019. Five independent reviewers assessed the methodological quality of the identified guidelines using the instruments “Appraisal of Guidelines for Research and Evaluation II” (AGREE II) and AGREE-REX (Recommendation EXcellence). Descriptive analysis was performed and subgroup differences were explored with the Kruskal–Wallis (H) test. The relationship between the individual domains and items of the instruments were examined using Spearman’s correlation.
Results
Five guidelines published from 2014 to 2018 by consortia of the United States of America, Canada and the United Kingdom (UK) were included. The highest scores were obtained by the UK guideline fulfilling 48–86% of criteria in AGREE II and 30–60% for AGREE-REX. All guidelines showed deficiencies in the domains “editorial independence”, “applicability”, and “recommendation”. Subgroup differences were identified only for the domain “editorial independence”.
Conclusion
The UK guideline achieved the highest scores with both instruments and may serve as a basis for future guideline development in UM. The domains “editorial independence”, “recommendation”, and “applicability” were identified as methodological weaknesses and require particular attention and improvement in future guidelines.
Similar content being viewed by others
Introduction
Uveal melanoma (UM) represents one of the most common ocular malignancies and accounts for about 5% of all melanomas. Primary tumors originate from the pigment cells of the choroid layer, the ciliary body or iris of the eye (Chattopadhyay et al. 2016). With an incidence of 4–7 cases per million in Europe, it is much rarer than cutaneous melanoma (Mallone et al. 2012). Typical driver mutations of cutaneous melanoma in the BRAF and NRAS genes are not found in UM. Instead, more than 80% harbour mutations in the guanine nucleotide binding protein Q polypeptide (GNAQ) and alpha-11 (GNA11) genes (Onken et al. 2008; Van Raamsdonk et al. 2009, 2010), leading to constitutive activation of the MAPK signalling pathway (Shoushtari and Carvajal 2014). Primary disease can be effectively controlled by several local therapy options, however, more than 50% of all UM patients develop distant metastases, predominantly to liver and lungs (Bedikian 2006). Therefore, several liver-directed treatment approaches have been developed, but failed to demonstrate an overall survival benefit (Agarwala et al. 2014). Once UM becomes metastatic, therapy options are limited and have been adopted mostly from cutaneous melanoma despite its clinical and genetic heterogeneity (Heppt et al. 2017b; Steeb et al. 2018).
Clinicians usually rely on evidence-based clinical guidelines for decision-making. Clinical practice guidelines include statements and recommendations intended to optimise patient care, which are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options (Graham et al. 2011). Several organizations from dermatological, oncological and ophthalmological societies have published guidelines for the treatment of UM (Nathan et al. 2015; Simpson et al. 2014; Weis et al. 2016). The management of UM is subject to country-specific health care conditions, which must be taken into consideration. As more and more guidelines on UM are being published, users often face multiple guidelines on the same topic, available from different consortia. Numerous methodologies have been developed for the assessment of guidelines (Brouwers et al. 2019; Rico Iturrioz et al. 2004; Zeng et al. 2015). Among these, the most widely applied and validated assessment tool is the “Appraisal of Guidelines for Research and Evaluation (AGREE) II”, which was also favoured by WHO (Dans and Dans 2010; Vlayen et al. 2005). AGREE II was published in 2009 as a revised version of the original AGREE instrument issued in 2001. It comprises 23 items grouped into 6 domains and 2 overall assessment items (Dans and Dans 2010). Recently, “AGREE-REX: Recommendation Excellence” has been launched as a complement to the AGREE II (AGREE-REX Research Team 2019). AGREE-REX is a newly developed tool for the evaluation of the clinical credibility and implementability of practice guidelines and a strategy to inform their development and reporting. It consists of 9 items grouped into 3 domains as well as 2 overall assessment items.
In this article, we critically appraise UM guidelines, which were identified in a systematic literature search and determine their methodological quality using the instruments AGREE II and AGREE-REX. Identifying possible weaknesses and strengths may help to improve future guideline work for this orphan disease and set a framework for an improved future treatment guideline on UM.
Methods
Eligibility criteria
Published national and international guidelines on UM were eligible for our appraisal. To provide an appraisal of the most recent and up-to-date guidelines, we only included those that have been published within the previous 5 years. Besides, guidelines had to be published in English or German language. As UM is an orphan disease, we included all guidelines irrespective of their methodological level and their development process. Hence, we also included informal expert statements that are neither based on a systematic assessment of the literature nor on a structured consensus process.
Search strategy and guideline selection
A systematic search for guidelines was carried out in guideline databases, including multidisciplinary guideline providers and subject-specific guideline providers (Supplementary Table S1). The key search terms included “uveal melanoma”, “ocular melanoma”, “iris melanoma”, “choroidal melanoma”, “ciliary body melanoma” and the German translation “Aderhautmelanom”. Additionally, Medline and Embase (both via Ovid) were searched until 27 May 2019. The detailed search strategy is presented in Supplementary Table S2. After the elimination of double hits, two authors (MVH, TS) independently screened the titles and abstracts of the records that were identified in the databases for eligibility. For records that were considered potentially relevant, the full-text guidelines were obtained, and the inclusion and exclusion criteria were applied. Whenever discrepancies arose, resolution was achieved by discussion with a third independent author (CB).
Data extraction and rating of the guidelines
Information on each included guideline regarding title, national authority/author, country of origin, publication date, methodological approach and scope were collected and summarized by two authors independently (TS, MVH).
AGREE II was used by five independent reviewers to assess the methodological quality of each guideline identified in the search on a 7-point scale ranging from 1 (strongly disagree) to 7 (strongly agree). Six domains with 23 items were assessed, including: scope and purpose (domain 1), stakeholder involvement (domain 2), rigor of development (domain 3), clarity of presentation (domain 4), applicability (domain 5), and editorial independence (domain 6). Based on the scores of the 6 domains, overall assessment was obtained to assess the quality of the guidelines. The different domains were followed by a general judgement of the guideline’s overall quality considering the evaluated criteria on a 7-point scale from “lowest possible quality” to “highest possible quality”. Furthermore, the evaluator was asked for an answer on the statement “I would recommend this guideline for use” (“yes”, “yes, with modifications” and “no”). The evaluations were performed independently and blinded towards the other evaluators’ assessments using the platform provided by AGREE (my agree plus on https://www.agreetrust.org/).
As a complement to AGREE II, the instrument AGREE-REX was used for the evaluation of the domains clinical credibility and implementability of the guidelines. AGREE-REX includes the 3 domains clinical applicability (domain 1), values and preferences (domain 2) and implementability (domain 3) with 9 items that must be considered to ensure that guideline recommendations are of high quality. This instrument was used by the same five independent authors who rated the quality of the credibility and implementability on a 7-point scale ranging from 1 (lowest quality) to 7 (highest quality). Furthermore, the evaluator was asked for an answer on the recommendation of this guideline in the appropriate context or in the reviewers’ context. The evaluations were performed independently and blinded towards the other evaluators’ assessments using an internally piloted data extraction spreadsheet using Microsoft Excel 2010.
Analysis
Domain scores were calculated as suggested by the AGREE II and AGREE-REX instructions as a sum of the scores of all evaluators’ assessments of the individual items in the domain for both instruments. The total scores for each domain were then expressed as a percentage of the maximum possible score for that domain. Hence, the range of possible evaluations was 0–100%, with 0% and 100% representing the worst and best possible rating for each domain, respectively.
Statistical analyses were conducted using SPSS (IBM SPSS Statistics version 24, IBM Corporation, Armonk, NY, USA). Descriptive analyses included mean (± standard deviation, SD) or median and interquartile ranges (IQR). Subgroup differences were explored with the Kruskal–Wallis (H) test. The relationship between the individual domains were examined using Spearman’s correlation. A significance level of 0.05 was considered statistically significant. The interrater agreement of the five reviewers was determined using Fleiss’ Kappa (Landis and Koch 1977).
Results
Guideline identification
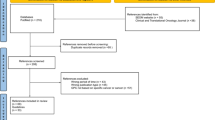
Our search in the databases and additional references revealed 1060 records (Fig. 1). After title and abstract screening and removal of duplicates, 13 records underwent full-text review. Two records were excluded since they were published before 2014 (Nag et al. 2003; Skalicky et al. 2008) and one was only available in French language (Mathis et al. 2018). Besides, one guideline was in development at the time of our search (National Institute for Health and Care Excellence 2019) and one more duplicate was identified (https://www.ncbi.nlm.nih.gov/books/NBK66047/). Another record was a comment on a guideline and was, therefore, excluded (Barker and Salama 2018). Furthermore, two guidelines were predominantly developed for the management of cutaneous melanoma and either did not address UM or did not cover it extensively or specifically enough to allow for an adequate appraisal with the chosen instruments (Leitlinienprogramm Onkologie 2019; Scottish Intercollegiate Guidelines Network 2017). Hence, five relevant guidelines were included in this comparison (National Cancer Institute 2019; Nathan et al. 2015; National Comprehensive Cancer Network 2018; Simpson et al. 2014; Weis et al. 2016). The publication date of the guidelines ranged from 2014 to 2018. Guidelines were available from consortia of the United States of America (n = 3), Canada (n = 1), and United Kingdom (UK) (n = 1) (Table 1). The majority of guidelines covered various aspects on UM, whereas one guideline exclusively focused on plaque brachytherapy of UM (Simpson et al. 2014). All included guidelines used distinct approaches to grade the level of evidence and to express the strength of their recommendations. The guideline assessment took place from June 2019 to August 2019. We determined a Fleiss’ Kappa of 0.088 (95% CI 0.069–0.108), indicating a slight overall interrater agreement concerning the assessment by AGREE II and AGREE-REX (Landis and Koch 1977).
AGREE II
Scope and purpose
This domain assesses whether the main objectives and questions of the guidelines and whether the population to whom the guideline is meant to apply were specifically described. It achieved an average score of 4.73 (± 1.55) (Supplementary Table S3). The guideline from the UK scored highest and fulfilled 82% of criteria whereas the guideline from the National Comprehensive Cancer Network (NCCN) from the US achieved only 35% of criteria (Fig. 2, 3a).
Stakeholder involvement
This domain evaluates whether the guideline was developed by appropriate stakeholders and represents the views of its intended users. Furthermore, it covers whether the target users of the guideline were clearly defined. The mean score of this domain was 4.17 (± 1.65) and the fulfilled values ranged from 71% for the UK guideline to intermediate values for the remaining guidelines (range 41–53%) (Fig. 2).
Rigor of development
This domain is about the methodological approaches of the guidelines and evaluates whether the identification of the evidence for the guideline was performed using systematic and transparent methods. Besides, this domain also assessed whether there was an explicit link between the recommendations and the supporting evidence, whether the guidelines had been externally reviewed and if a procedure for updating the guideline was available. It achieved an average rating of 3.92 (± 1.38). Besides, the UK guideline was evaluated by the reviewers to be of best methodological quality (77% of items fulfilled) in contrast to the remaining guidelines varying in terms of items fulfilled from 28 to 45%.
Clarity and presentation
This domain included the presentation and format of guidelines, i.e. whether the recommendations were specific and ambiguous, if key recommendations were easily identifiable and whether different options for the management of the condition were clearly presented. In general, all guidelines achieved high levels of fulfillment (5.01 ± 1.62) with the UK guideline being evaluated best with 86% of fulfilled criteria.
Application
This domain covers the processes related to guideline implementation, for instance facilitators, barriers, additional material provided and whether monitoring and/or auditing criteria were presented. This domain showed a mean score of 2.85 (± 1.43). The UK guideline achieved the highest score in comparison to the remaining guidelines (48%). The guidelines by Weis et al. and Simpson et al. achieved lowest possible scores with 17% and 18%, respectively.
Editorial independence
This domain focuses on funders and competing interests of experts involved in guideline development, i.e. whether competing interests of the development group were recorded and addressed. This domain achieved the lowest score with a mean of 2.66 (± 1.38). However, the UK guideline still achieved 73% of fulfilled criteria. The five identified guidelines significantly differed from each other (p = 0.038). No further subgroup differences were identified.
Overall assessment
This assessment rates the overall quality of the guidelines and whether the guideline would be recommended for use in practice. Overall, the guidelines achieved a mean score with 4.04 (± 1.06). The individual fulfilled criteria ranged from 40% (NCI guideline) to 70% (UK guideline). Hence, according to the assessment, only the UK guideline would be recommended whereas the remaining 4 guidelines are recommended with modifications only.
AGREE-REX
Clinical applicability
This domain evaluates whether the guideline is evidence-based (i.e. based on a thorough review and assessing potential bias) as well as the degree to which the recommendations are applicable to the guideline’s target users’ practice context and patients. The guideline from the UK Melanoma group achieved the highest percentage value with 59%, and the guideline by Weis et al. the lowest with 36% (Fig. 3b, Supplementary Table S4). The mean score in this domain was 3.83 ± 1.37 (Fig. 2).
Values and preferences
This domain comprises four different items and refers to the relative importance that target users, patients, policy/decision-makers as well as guideline developers place on the outcomes of interest. Their values and preferences are important in guideline development as they influence whether recommendations are acceptable and adopted into practice. Therefore, this domain assesses if their views and its impact had been explored and considered in the formulation of the recommendations. The mean score was 3.28 ± 0.97 for this domain. All guidelines achieved a value ranging from 32 to 35%, except for the UK guideline which achieved 55% in this domain.
Implementability
The implementability domain includes the items “purpose” and “local application and adoption”. This domain assesses the suitability of the guideline recommendations for patients/populations, and/or the health care systems in which they are being implemented and if the degree of change from current practice was addressed. Furthermore, the guideline should articulate relevant factors important to its successful dissemination. Besides, the purpose item evaluates weather guideline recommendations aligned with the implementation goals of the guidelines. Again, the UK guideline achieved highest results with 60%, followed by the guideline of the American Brachytherapy Society (47%). The mean score was 3.66 ± 1.42.
Recommendations
This domain assessed whether the raters would recommend this guideline either in the appropriate context as well as in the rater’s context. Overall, none of the guidelines achieved sufficient values as they ranged from 17 to 30% and as the mean score was 2.50 ± 1.30.
Correlations of the AGREE II and AGREE-REX domains
The majority of AGREE II domains significantly correlated with each other (Table 2). The domain “overall assessment” was significantly positively correlated with all other domains. “Stakeholder involvement” was highly positively correlated with the domain “overall assessment” (r = 0.81), the domain “scope and purpose” (r = 0.76), “clarity of presentation” (r = 0.48) and “rigor of development” (r = 0.48). Besides this, “clarity of presentation” was positively associated with “rigor of development” (r = 0.43) and “applicability” (r = 0.57). The AGREE-REX domains were all positively and statistically significantly correlated with each other, except for the domain “values and preferences” and the additional recommendation item.
Discussion
UM is an orphan cancer condition of high unmet clinical need. Although UM differs from cutaneous melanoma both clinically and biologically, treatment options for advanced stages have largely been adopted from cutaneous melanoma, yet with much lower response rates and at the cost of high treatment-related toxicity (Heppt et al. 2017a,2019). In addition, numerous high-quality guidelines are available for the care of cutaneous melanoma, which facilitate the clinical and diagnostic algorithms in a standardized fashion (Pflugfelder et al. 2013; Swetter et al. 2019). In contrast, few studies have been published for UM and large randomized controlled trials are widely lacking. This makes it difficult to demonstrate the clinical effectiveness of interventions and to create a solid framework for evidence-based treatment decisions. A further barrier comes from the fact that the care of patients with UM occurs in a highly interdisciplinary setting, involving ophthalmologists, medical oncologists, interventional radiologists and dermato-oncologists. Patients suffering from rare cancers may have limited access to specialized cancer centers, potentially resulting in sub-optimal management and outcomes. These considerations highlight the value of high-quality guidelines in rare cancers even in the absence of high-quality evidence and underlines that special strategies need to be employed to synthesize evidence that is compatible with rigorous quality standards of guidelines (Pai et al. 2019).
In this study, we identified 5 guidelines for UM in a systematic literature search published within the previous 5 years and evaluated their methodological quality. Surprisingly, all of them were evidence-based and none was developed based on an expert consensus only. This contrasts with the fact that the domain “rigor of development” achieved rather low values (25–50%) in 4 out of 5 guidelines. This domain considers inter alia if systematic methods were applied to identify the evidence and if criteria for the literature search were clearly described and transparent. Thus, the majority of guidelines fell short of complying with these criteria. Similarly, the assessors gave low to intermediate ratings (25–50%) to the domains “overall recommendation”, “clinical applicability”, “values and preference”, and “implementability”, warranting improvement and special attention in future guideline efforts. The lowest values (≤ 25%) were observed for “applicability”, “editorial independence”, and “recommendation”. Applicability refers to how facilitators and barriers of the guideline application were discussed and if tools for monitoring or auditing the recommendations were provided. These parameters were not sufficiently addressed in any of the guidelines, possibly explaining the low ratings of this domain. The domain editorial independence aims at ensuring that the guideline is editorially independent from the funding body and that all conflicts of interest of guideline development members are correctly disclosed. The ratings for this domain were conspicuously low among the evaluated guidelines. On the one hand, it is possible that the editorial independency was present, but simply not indicated correctly. On the other hand, we cannot rule out the possibility that funding parties may have exerted an influence on the content of the guidelines or that the members of the guidelines group may have had conflicts of interest. Developing methodologically sound guidelines is costly and requires major financial, organizational and human resources. Especially in the case of orphan diseases such as UM, public and independent funding may not be available due to a low public awareness. Finally, consistently low values were achieved for “recommendation”, i.e. the usability of the recommendations made for a specific health-care context. Apparently, there was a major mismatch between the recommendations of the 5 guidelines with the specific care context of the assessor. Thus, the medical needs both of treating physicians and patients must be explored and aligned with the guideline recommendations, as has been performed for other orphan conditions (Boffin et al. 2018). Importantly, our evaluation did not evaluate the recommended therapies in the respective guideline but instead focused on their methodological quality.
Altogether, this analysis demonstrates that guidelines for UM as prime example of an orphan cancer have room for methodological improvement. The published UK guideline showed the best ratings in this study, suggesting that it may serve as good adaption basis for future guideline projects. In particular, the domains “applicability”, “editorial independence”, and “recommendation” should be improved to ensure that the guidelines give independent and context-specific guidance for clinicians.
References
Agarwala SS, Eggermont AM, O'Day S, Zager JS (2014) Metastatic melanoma to the liver: a contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer 120:781–789. https://doi.org/10.1002/cncr.28480
AGREE-REX Research Team (2019). The Appraisal of Guidelines Research & Evaluation—Recommendation EXcellence (AGREE-REX) [Electronic version]. https://www.agreetrust.org/wp-content/uploads/2019/04/AGREE-REX-2019.pdf. Accessed 18 Nov 2019
Barker CA, Salama AK (2018) New NCCN guidelines for uveal melanoma and treatment of recurrent or progressive distant metastatic melanoma. J Natl Compr Cancer Netw 16:646–650. https://doi.org/10.6004/jnccn.2018.0042
Bedikian AY (2006) Metastatic uveal melanoma therapy: current options. Int Ophthalmol Clin 46:151–166
Boffin N, Swinnen E, Wens J, Urbina M, Van der Heyden J, Van Casteren V (2018) General practice care for patients with rare diseases in Belgium A Cross-Sectional Survey. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15061180
Brouwers MC, Florez ID, McNair SA, Vella ET, Yao X (2019) Clinical practice guidelines: tools to support high quality patient care. Semin Nucl Med 49:145–152. https://doi.org/10.1053/j.semnuclmed.2018.11.001
Chattopadhyay C et al (2016) Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 122:2299–2312. https://doi.org/10.1002/cncr.29727
Dans AL, Dans LF (2010) Appraising a tool for guideline appraisal (the AGREE II instrument). J Clin Epidemiol 63:1281–1282. https://doi.org/10.1016/j.jclinepi.2010.06.005
Graham R, Mancher M, Wolman MD, Greenfield S, Steinberg E (2011) Clinical practice guidelines we can trust. National Academies Press, Washington DC. https://doi.org/10.17226/13058
Heppt MV et al (2017a) Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur J Cancer 82:56–65. https://doi.org/10.1016/j.ejca.2017.05.038
Heppt MV et al (2017b) Immune checkpoint blockade for unresectable or metastatic uveal melanoma: a systematic review. Cancer Treat Rev 60:44–52. https://doi.org/10.1016/j.ctrv.2017.08.009
Heppt MV et al (2019) Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunother Cancer 7:299. https://doi.org/10.1186/s40425-019-0800-0
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Mallone S et al (2012) Descriptive epidemiology of malignant mucosal and uveal melanomas and adnexal skin carcinomas in Europe. Eur J Cancer 48:1167–1175. https://doi.org/10.1016/j.ejca.2011.10.004
Mathis T et al (2018) Management of uveal melanomas, guidelines for oncologists. Bull Cancer 105:967–980. https://doi.org/10.1016/j.bulcan.2018.07.011
Nag S, Quivey JM, Earle JD, Followill D, Fontanesi J, Finger PT, American Brachytherapy S (2003) The American Brachytherapy Society recommendations for brachytherapy of uveal melanomas. Int J Radiat Oncol Biol Phys 56:544–555
Nathan P et al (2015) Uveal melanoma UK national guidelines. Eur J Cancer 51:2404–2412. https://doi.org/10.1016/j.ejca.2015.07.013
National Cancer Institute (2019) Intraocular (Uveal) Melanoma treatment (PDQ®)–health professional version. https://www.cancer.gov/types/eye/hp/intraocular-melanoma-treatment-pdq#_84. Accessed 19 Nov 2019
National Comprehensive Cancer Network (NCCN) (2018) Clinical Practice Guidelines in Oncology Uveal Melanoma, Version I. 2018
National Institute for Health and Care Excellence (2019) IMCgp100 for treating metastatic uveal melanoma (ID1441). https://www.nice.org.uk/guidance/indevelopment/gid-ta10428. Accessed 20 Nov 2019
Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, Harbour JW (2008) Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest Ophthalmol Vis Sci 49:5230–5234. https://doi.org/10.1167/iovs.08-2145
Leitlinienprogramm Onkologie (2019) (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik, Therapie und Nachsorge des Melanoms, Langversion 3.2, 2019, AWMF Registernummer: 032/024OL, https://www.leitlinienprogramm-onkologie.de/leit-linien/melanom/. Accessed 18 Nov 2019
Pai M et al (2019) Strategies for eliciting and synthesizing evidence for guidelines in rare diseases. BMC Med Res Methodol 19:67. https://doi.org/10.1186/s12874-019-0713-0
Pflugfelder A et al (2013) Malignant melanoma S3-guideline "diagnosis, therapy and follow-up of melanoma". J Dtsch Dermatol Ges 11(Suppl 6):1–116. https://doi.org/10.1111/ddg.12113_suppl
Rico Iturrioz R, Gutierrez-Ibarluzea I, Asua Batarrita J, Navarro Puerto MA, Reyes Dominguez A, Marin Leon I, de la Blanca Briones EP (2004) Assessment of clinical practice guidelines evaluation. Scales and criteria. Rev Esp Salud Publica 78:457–467. https://doi.org/10.1590/s1135-57272004000400004
Scottish Intercollegiate Guidelines Network (SIGN) (2017). Cutaneous melanoma (SIGN publication no. 146). https://www.sign.ac.uk/assets/sign146.pdf. Accessed 20 Dec 2019
Shoushtari AN, Carvajal RD (2014) GNAQ and GNA11 mutations in uveal melanoma. Melanoma Res 24:525–534. https://doi.org/10.1097/CMR.0000000000000121
Simpson ER et al (2014) The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy 13:1–14. https://doi.org/10.1016/j.brachy.2013.11.008
Skalicky SE, Holt PE, Giblin M, Taylor S, Conway RM (2008) Australian cancer network clinical practice guidelines for the management of ocular and periocular melanoma: an evidence-based literature analysis. Clin Experiment Ophthalmol 36:646–658. https://doi.org/10.1111/j.1442-9071.2008.01815.x
Steeb T, Wessely A, Ruzicka T, Heppt MV, Berking C (2018) How to MEK the best of uveal melanoma: a systematic review on the efficacy and safety of MEK inhibitors in metastatic or unresectable uveal melanoma. Eur J Cancer 103:41–51. https://doi.org/10.1016/j.ejca.2018.08.005
Swetter SM et al (2019) Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol 80:208–250. https://doi.org/10.1016/j.jaad.2018.08.055
Van Raamsdonk CD et al (2009) Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457:599–602. https://doi.org/10.1038/nature07586
Van Raamsdonk CD et al (2010) Mutations in GNA11 in uveal melanoma. N Engl J Med 363:2191–2199. https://doi.org/10.1056/NEJMoa1000584
Vlayen J, Aertgeerts B, Hannes K, Sermeus W, Ramaekers D (2005) A systematic review of appraisal tools for clinical practice guidelines: multiple similarities and one common deficit. Int J Qual Health Care 17:235–242. https://doi.org/10.1093/intqhc/mzi027
Weis E et al (2016) Management of uveal melanoma: a consensus-based provincial clinical practice guideline. Current Oncology 23:e57–64. https://doi.org/10.3747/co.23.2859
Zeng X et al (2015) The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8:2–10. https://doi.org/10.1111/jebm.12141
Acknowledgements
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were conducted by TS, MVH, FT, MS, KMH; analysis were performed by TS and MVH. The first draft of the manuscript was written by TS and MVH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Steeb, T., Hayani, K.M., Förster, P. et al. Guidelines for uveal melanoma: a critical appraisal of systematically identified guidelines using the AGREE II and AGREE-REX instrument. J Cancer Res Clin Oncol 146, 1079–1088 (2020). https://doi.org/10.1007/s00432-020-03141-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03141-w