Abstract
Purpose
We attempt to assess the impact of hepatis-B virus (HBV) status on the prognosis of chronic lymphocytic leukemia (CLL) using a Chinese case cohort.
Methods
Five hundred and one consecutive newly diagnosed subjects with CLL were enrolled in this case cohort. HBV infection was defined as hepatitis B surface antigen (HBsAg) positive or hepatitis-B core antibody (HBcAb) positive. Univariate and stepwise multivariate Cox regression analyses were used to screen the prognostic risk factors associated with the end point of time-to-treatment (TTT) or overall survival (OS). Bootstrap re-sampling method was used to evaluate the model’s internal validity. The discriminative ability of the models was evaluated using time-dependent receiver–operator characteristic (ROC) curves and corresponding areas under the curve (AUC).
Results
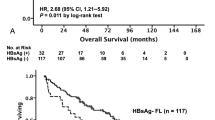
One hundred and twenty-one subjects (24%) among 501 patients were HBV positive. HBV infection was an independent predictor for the prognosis of TTT (HR = 1.37; 95% CI 1.04–1.80) or OS (HR =2.85; 95% CI 1.80–4.52). The AUCs for HBV infection were 0.62 (95% CI 0.58–0.66) for TTT and 0.69 (95% CI 0.66–0.72) for OS, respectively. When we combined HBV infection with the traditional clinical and biological factors, significant improvements for model’s discrimination were observed for TTT [AUC: 0.81 (95% CI: 0.77–0.85) vs. 0.78 (95% CI: 0.74–0.82), P < 0.001] and OS [AUC: 0.81 (95% CI 0.76–0.86) vs. 0.76 (95% CI 0.71–0.82), P < 0.001). Further bootstrap re-sampling method revealed good internal consistence for the final optimal models (Average AUC: 0.78 for TTT and 0.79 for OS based on 1000 bootstraps).
Conclusions
Our results indicated that HBV infection should be served as an important risk predictor for prognosis of CLL (TTT and OS).


Similar content being viewed by others
References
Binet JL, Auquier A, Dighiero G et al (1981) A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 48:198–206
Bulian P, Rossi D, Forconi F et al (2012) IGHV gene mutational status and 17p deletion are independent molecular predictors in a comprehensive clinical-biological prognostic model for overall survival prediction in chronic lymphocytic leukemia. J Transl Med 10:18
Chen MH, Hsiao LT, Chiou TJ et al (2008) High prevalence of occult hepatitis B virus infection in patients with B cell non-Hodgkin’s Lymphoma. Ann Hematol 87:475–480
Cheson BD, Bennett JM, Grever M et al (1996) National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 87:4990–4997
Cortese D, Sutton LA, Cahill N et al (2014) On the way towards a ‘CLL prognostic index’: focus on TP53, BIRC3, SF3B1, NOTCH1 and MYD88 in a population-based cohort. Leukemia 28:710–713
Cramer P, Hallek M (2011) Prognostic factors in chronic lymphocytic leukemia-what do we know? Nat Rev Clin Oncol 8:38–47
de Sanjose S, Benavente Y, Vajdic CM et al (2008) Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortiumm. Clin Gastroenterol Hepatol 6:451–458
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Dighiero G, Hamblin TJ (2008) Chronic lymphocytic leukaemia. Lancet 371:1017–1029
Fung J, Lai CL, Yuen MF (2009) Hepatitis B and C virus-related carcinogenesis. Clin Microbiol Infect 15:964–970
Gentile M, Mauro FR, Rossi D et al (2014) Italian external and multicentric validation of the MD Anderson Cancer Center nomogram and prognostic index for chronic lymphocytic leukaemia patients: analysis of 1502 cases. Br J Haematol 167:224–232
Gibson TM, Morton LM, Shiels MS et al (2014) Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS 28:2313–2318
Gopal S, Patel MR, Yanik EL et al (2013) Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst 105:1221–1229
Grulich AE, van Leeuwen MT, Falster MO et al (2007) Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 370:59–67
Haferlach C, Dicker F, Weiss T et al (2010) Toward a comprehensive prognostic scoring system in chronic lymphocytic leukemia based on a combination of genetic parameters. Genes Chromosom Cancer 49:851–859
Hallek M, Cheson BD, Catovsky D et al (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic leukemia updating the National Cancer Institue-Working Group 1996 guidelines. Blood 111:5446–5456
Heagerty PJ, Lumley T, Pepe MS (2000) Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 56:337–344
Ji D, Cao J, Hong X et al (2010) Low incidence of hepatitis B virus reactivation during chemotherapy among diffuse large B-cell lymphoma patients who are HBsAg-negative/ HBcAb-positive: a multicenter retrospective study. Eur J Haematol 85:243–250
Kay NE, O’Brien SM, Pettitt AR et al (2007) The role of prognostic factors in assessing ‘high-risk’ subgroups of patients with chronic lymphocytic leukemia. Leukemia 21:1885–1891
Letestu R, Lévy V, Eclache V et al (2010) Prognosis of Binet stage A chronic lymphocytic leukemia patients: the strength of routine parameters. Blood 116:4588–4590
Liang XF, Chen YS, Wang XJ et al (2005) A study on the sero-epidemiology of hepatitis B in Chinese population aged over 3-years old: the report from Chinese Center for Disease Control and Prevention. Chin J Epidemiol 26:655–658
Liaw YF, Chu CM (2009) Hepatitis B virus infection. Lancet 373:582–592
Liu J, Fan D (2007) Hepatitis B in China. Lancet 369:1582–1583
Mele A, Pulsoni A, Bianco E et al (2003) Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter case-control study. Blood 102:996–999
Minuk GY, Lerner B, Gibson SB et al (2014) Hepatitis B and C virology infections in patients with chronic lymphocytic leukemia. Can J Gastroenterol Hepatol 28:131–134
Molica S, Giannarelli D, Mirabelli R et al (2016) Unavailability of thymidine kinase does not preclude the use of German comprehensive prognostic index: results of an external validation analysis in early chronic lymphocytic leukemia and comparison with MD Anderson Cancer Center model. Eur J Haematol 96:72–77
Pflug N, Bahlo J, Shanafelt TD et al (2014) Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood 124:49–62
Rai KR, Sawitsky A, Cronkite EP et al (1975) Clinical staging of chronic lymphocytic leukemia. Blood 46:219–234
Sauerbrei W, Schumacher M (1992) A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med 11:2093–2109
Serdlow SH, Campo E, Harris NL (2008) WHO classification of tumours of haematopoietic and lymphoid tissue, 4th. IARC Press, Lyon
Thorley-Lawson DA, Gross A (2004) Persistence of the Epstein-Barr Virus and the origins of Associated Lymphomas. N Engl J Med 350:1328–1337
Trajkova S, Cevreska L, Pivkova-Veljanovska A et al (2013) Multivariable model consisting of clinical and biological markers for time to first treatment in CLL patients: preliminary results from single centre experience. Prilozi 34:39–48
Tsai WL, Chung RT (2010) Virology hepatocarcinogenesis. Oncogene 29:2309–2324
Visentin A, Facco M, Frezzato F et al (2015) Integrated CLL Scoring System, a New and Simple Index to Predict Time to Treatment and Overall Survival in Patients With Chronic Lymphocytic Leukemia. Clin Lymphoma Myeloma Leuk 15:612–620
Wang YH, Fan L, Wang L et al (2012) Efficacy of prophylactic lamivudine to prevent hepatitis B virus reactivation in B-cell lymphoma treated with rituximab-containing chemotherapy. Support Care Cancer 21:1265–1271
Yeo W, Chan TC, Leung NW et al (2009) Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 27:605–611
Acknowledgements
This study was founded by National Natural Science Foundation of China (81370657, 81470328, 81600130, 81770166, 81720108002), Jiangsu Province’s Medical Elite Programme (ZDRCA2016022), Project of National Key Clinical Specialty, National Science & Technology Pillar Program (2014BAI09B12), Jiangsu Provincial Special Program of Medical Science (BL2014086 and BE2017751) and National Science and Technology Major Project (2017ZX09304032).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
These authors have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, JH., Gao, R., Dai, JC. et al. The prognostic role of HBV infection in chronic lymphocytic leukemia. J Cancer Res Clin Oncol 144, 1309–1315 (2018). https://doi.org/10.1007/s00432-018-2663-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2663-z