Abstract
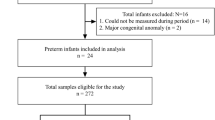
The purpose of this paper is to verify whether the concentrations of caffeine in saliva are comparable to serum concentrations in preterm infants who are treated with caffeine for apnea of prematurity. This is a prospective observational study. Eligible participants were newborn infants < 37 weeks of gestational age treated with oral or intravenous caffeine for apnea of prematurity. Two paired samples of saliva and blood were collected per patient. Tube solid-phase microextraction coupled online to capillary liquid chromatography with diode array detection was used for analysis. A total of 47 infants with a median gestational age of 28 [26–30] weeks and a mean of 1.11 ± 0.4 kg of birth weight. Median postmenstrual age, when samples were collected, was 31 [29–33] weeks. Serum caffeine median levels of 19.30 μg/mL [1.9–53.90] and salivary caffeine median levels of 16.36 μg/mL [2.20–56.90] were obtained. There was a strong positive Pearson’s correlation between the two variables r = 0.83 (p < 0.001).
Conclusion: The measurement of salivary caffeine concentrations after intravenous or oral administration offers an alternative to serum caffeine monitoring in apnea of prematurity. Measurement of salivary concentration minimizes blood draws, improves blood conservation, and subsequently minimizes painful procedures in premature infants.
What is Known: • Salivary sampling may be useful when is applied to extremely low birth weight infant, in whom blood sampling must be severely restricted. | |
What is New: • The measurement of caffeine salivary concentrations after intravenous or oral administration offers an alternative to serum caffeine monitoring in apnoea of prematurity. • Salivary sampling may be a valid non-invasive alternative that could be used to individualize and optimize caffeine dose. |




Similar content being viewed by others
Abbreviations
- AOP:
-
Apnea of prematurity
- GA:
-
Gestational age
- HPLC:
-
High-pressure liquid chromatography
- IV:
-
Intravenous
- NICU:
-
Neonatal intensive care unit
- O:
-
Oral
- PMA:
-
Postmenstrual age
References
Paolillo P, Picone S (2013) Apnea of prematurity. J Pediatr Neonatal Individ Med JPNIM 2(2):e020213. https://doi.org/10.7363/020213
Eichenwald EC (2016) Committee on fetus and newborn, American Academy of Pediatrics. Apnea of Prematurity. Pediatrics 137(1). https://doi.org/10.1542/peds.2015-3757
Henderson-Smart DJ, De Paoli AG (2010) Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst Rev (12):CD000140. https://doi.org/10.1002/14651858.CD000140.pub2
Pacifici GM (2014) Clinical pharmacology of caffeine citrate in preterm infants. MedicalExpress 1(5):243–250. https://doi.org/10.5935/MedicalExpress.2014.05.06
Peyona-Summary of product characteristic. EMA-Europe. https://www.ema.europa.eu/en/documents/product-information/peyona-epar-product-information_en.pdf. Accessed 17 Jan 2022
Moschino L, Zivanovic S, Hartley C, Trevisanuto D, Baraldi E, Roehr CC (2020) Caffeine in preterm infants: where are we in 2020? ERJ Open Res 6(1). https://doi.org/10.1183/23120541.00330-2019
Mohammed S, Nour I, Shabaan AE, Shouman B, Abdel-Hady H, Nasef N (2015) High versus low-dose caffeine for apnea of prematurity: a randomized controlled trial. Eur J Pediatr 174(7):949–956. https://doi.org/10.1007/s00431-015-2494-8
Vliegenthart R, Miedema M, Hutten GJ, van Kaam AH, Onland W (2018) High versus standard dose caffeine for apnoea: a systematic review. Arch Dis Child Fetal Neonatal Ed 103(6):F523–F529. https://doi.org/10.1136/archdischild-2017-313556
Lista G, Fabbri L, Polackova R et al (2016) The real-world routine use of caffeine citrate in preterm infants: a European postauthorization safety study. Neonatology 109(3):221–227. https://doi.org/10.1159/000442813
Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A (2008) Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Ther Drug Monit 30(6):709–716. https://doi.org/10.1097/FTD.0b013e3181898b6f
Hutchinson L, Sinclair M, Reid B, Burnett K, Callan B (2018) A descriptive systematic review of salivary therapeutic drug monitoring in neonates and infants. Br J Clin Pharmacol 84(6):1089–1108. https://doi.org/10.1111/bcp.13553
Ponce-Rodríguez HD, García-Robles AA, Sáenz-González P, Verdú-Andrés J, Campíns-Falcó P (2020) On-line in-tube solid phase microextraction coupled to capillary liquid chromatography-diode array detection for the analysis of caffeine and its metabolites in small amounts of biological samples. J Pharm Biomed Anal 178:112914. https://doi.org/10.1016/j.jpba.2019.112914
Kuligowski J, Escobar J, Quintás G et al (2014) Analysis of lipid peroxidation biomarkers in extremely low gestational age neonate urines by UPLC-MS/MS. Anal Bioanal Chem 406(18):4345–4356. https://doi.org/10.1007/s00216-014-7824-6
Schmidt B, Roberts RS, Davis P et al (2006) Caffeine therapy for apnea of prematurity. N Engl J Med 354(20):2112–2121. https://doi.org/10.1056/NEJMoa054065
Lodha A, Seshia M, McMillan DD et al (2015) Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr 169(1):33–38. https://doi.org/10.1001/jamapediatrics.2014.2223
Patel RM, Leong T, Carlton DP, Vyas-Read S (2013) Early caffeine therapy and clinical outcomes in extremely preterm infants. J Perinatol 33(2):134–140. https://doi.org/10.1038/jp.2012.52
Schmidt B, Roberts RS, Davis P et al (2007) Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 357(19):1893–1902. https://doi.org/10.1056/NEJMoa073679
Lodha A, Entz R, Synnes A et al (2019) Early Caffeine Administration and Neurodevelopmental Outcomes in Preterm Infants. Pediatrics 143(1). https://doi.org/10.1542/peds.2018-1348
Saroha V, Patel RM (2020) Caffeine for preterm infants: fixed standard dose, adjustments for age or high dose? Semin Fetal Neonatal Med 25(6):101178. https://doi.org/10.1016/j.siny.2020.101178
Koch G, Datta AN, Jost K, Schulzke SM, van den Anker J, Pfister M (2017) Caffeine citrate dosing adjustments to assure stable caffeine concentrations in preterm neonates. J Pediatr 191:50-56.e1. https://doi.org/10.1016/j.jpeds.2017.08.064
Kumral A, Tuzun F, Yesilirmak DC, Duman N, Ozkan H (2012) Genetic basis of apnoea of prematurity and caffeine treatment response: role of adenosine receptor polymorphisms: genetic basis of apnoea of prematurity. Acta Paediatr Oslo Nor 1992 101(7):e299–303. https://doi.org/10.1111/j.1651-2227.2012.02664.x
Khanna NN, Bada HS, Somani SM (1980) Use of salivary concentrations in the prediction of serum caffeine and theophylline concentrations in premature infants. J Pediatr 96(3):494–499. https://doi.org/10.1016/S0022-3476(80)80708-6
Lee TC, Charles BG, Steer PA, Flenady V (1996) Saliva as a valid alternative to serum in monitoring intravenous caffeine treatment for apnea of prematurity. Ther Drug Monit 18(3):288–293. https://doi.org/10.1097/00007691-199606000-00012
de Wildt SN, Kerkvliet KT, Wezenberg MG et al (2001) Use of saliva in therapeutic drug monitoring of caffeine in preterm infants. Ther Drug Monit 23(3):250–254. https://doi.org/10.1097/00007691-200106000-00011
Chaabane A, Chioukh FZ, Chadli Z et al (2017) Therapeutic drug monitoring of caffeine in preterm infants: could saliva be an alternative to serum? Therapie 72(6):685–689. https://doi.org/10.1016/j.therap.2017.06.004
Tracy MB, Klimek J, Hinder M, Ponnampalam G, Tracy SK (2010) Does caffeine impair cerebral oxygenation and blood flow velocity in preterm infants? Acta Paediatr Oslo Nor 1992 99(9):1319–1323. https://doi.org/10.1111/j.1651-2227.2010.01828.x
Dobson NR, Liu X, Rhein LM et al (2016) Salivary caffeine concentrations are comparable to plasma concentrations in preterm infants receiving extended caffeine therapy. Br J Clin Pharmacol 82(3):754–761. https://doi.org/10.1111/bcp.13001
Acknowledgements
The authors would like to thank the staff and the nurses of the Neonatology Division for the dedication and support to this project. The authors thank Elena Ramirez Saenz and Luis González Batanero for their support in the writing and revision of language, grammar, and terminology.
Funding
The authors are grateful to VLC-BIOMED program, project 04-CAFE-CINETIC-VERDU-SAENZ-2016-A. ASG and MVT acknowledge RETICS funded by the PN 2018–2021 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (FEDER), reference RD16/0022/0001.
Author information
Authors and Affiliations
Contributions
AGR, ASG, MVT, JVA, JPA, CHP, and PSG were all involved in development of the study. AGR, ASG and PSG prepared the initial draft of the manuscript and set up the database infrastructure for the intervention. ACM performed statistical analyses. JVA and HDPR performed analytical sample procedures. All authors read, contributed to editing, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was reviewed and approved by the Institutional Review Board of our hospital (approval identification code: GIP-CAF-2017–01/408). Written informed consent was obtained from the parents of all patients before inclusion in the study.
Consent to participate
All legal representatives of patients consent their participation after receiving patient information and consents.
Conflict of interest
The authors declare no competing interests.
Disclaimer
The authors confirm that if their article is published in ANC, they will notify the relevant parties accordingly so that the preprint version can be linked to the published record.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was first posted on Research Square at the following address: https://www.researchsquare.com/article/rs-236907/v1.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
García-Robles, A., Solaz-García, Á., Verdú-Andrés, J. et al. The association of salivary caffeine levels with serum concentrations in premature infants with apnea of prematurity. Eur J Pediatr 181, 4175–4182 (2022). https://doi.org/10.1007/s00431-022-04628-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04628-z