Abstract
During the coronavirus disease 2019 (COVID-19) epidemic, many reports have indicated that children shed the virus longer than adults in stool, and that most of the children had mild or even asymptomatic infections, which increased the potential risk for feces to be a source of contamination and may play an important role in the spread of the virus. In this review, we collected relevant literature to summarize the duration of fecal viral shedding in children with COVID-19. We found that in about 60% of the cases, the fecal shedding time was between 28 and 42 days, which was much longer than that of adults. We further explored the possible reason for prolonged shedding and its the potential impact. The poor hand hygiene practices of children, their tendency to swallow sputum and/or saliva, the significant difference in expression of angiotensin-converting enzyme 2 (ACE2) in intestine between children and adults, and the variance in immune status and intestinal microbiome could be considered as potential casual agents of longer fecal viral shedding duration of children.
Conclusion: Children with COVID-19 show prolonged fecal shedding compared to adults. Several mechanisms may be involved in the longer fecal viral shedding. Viral shedding in the stool could be contributing to a possible route of transmission. Therefore, we think that further preventive measures in children should be taken to reduce the spread of the disease.
What is Known: • Children with COVID-19 are more likely to have asymptomatic infections and to experience mild symptoms. • Some patients continue to shed the virus in feces, despite respiratory samples testing negative. | |
What is New: • Children with COVID-19 carried a longer-term fecal viral shedding than adults. • The poor hand hygiene practices of children, their tendency to swallow sputum and/or saliva, the difference in expression of ACE2 in intestine between children and adults, and the variance in immune status and intestinal microbiome could be considered as potential casual agents of longer fecal viral shedding duration of children. |
Similar content being viewed by others
Introduction
COVID-19 continues to pose a global threat with the emergence of new variants. It has been reported that children with COVID-19 often had a milder disease course and they were possible sources of its spread [1]. Children have also reported to have prolonged shedding of syndrome coronavirus 2 (SARS-CoV-2) in feces compared to adults[2–4], which, combined with the possibility of fecal–oral transmission [5], lead to concerns that children may be potential sources of undetected community transmission. This study aims to summarize the existing data on the duration of fecal viral shedding in children with COVID-19 and explore the reasons for prolonged shedding and its potential effects.
Method
A systematic electronic databases search was performed in PubMed/MEDLINE and Web of Science using the search terms “COVID-19 or 2019-nCoV or SARS-CoV-2” and “pediatrics or children or infant or neonate or teenagers or adolescents” and “fecal or fecal or stool or rectal” between 2019 and the present time (i.e., January 6, 2022). In addition, the reference lists of all known primary and review articles were scrutinized to identify cited articles not captured by the electronic searches. Studies were included if they reported data on the duration of gastrointestinal viral shedding in children with COVID-19 in English.
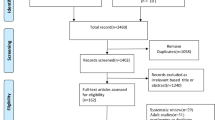
The initial search produced 153 potentially relevant articles. After removing duplicates and excluding irrelevant articles, 70 full-text articles were assessed. Articles were further excluded because of the following: (1) the articles did not present original data (n = 7); (2) reported cases with incomplete information (n = 7); (3) review articles, meta-analyses, perspectives, comments, opinion articles, and letters (n = 21); (4) studies including adult-only or including adults and children but not did not present sufficient data for children (n = 10); and (5) studies not written in English (Fig. 1).
Results
A total of 25 studies (n = 176 patients) were included in the final analysis [2–4, 6–27]. Among the selected studies, 14 (56%) were from China and 11 (44%) were from other countries (South Korea: three, Japan: one, Singapore: one, Italy: one, the Netherlands: one, Germany: one, India: one, and Iran: one). The number of cases enrolled in each study ranged from one to 49 and the age ranged from 7 days to 210 months. Of the 176 cases, 175 (99.4%) had a positive nasopharyngeal or throat swab (one case had positive stool specimens only). The duration of viral shedding via the respiratory route ranged from 0 day to at least 1 month, and the duration of gastrointestinal viral shedding ranged from 6 to 100 days. Several studies reported the duration of viral shedding as a range only, and thus, the mean value could not be calculated. The prevalence of all gastrointestinal symptoms was 24% (43/176), including diarrhea, vomiting, abdominal pain, liver function abnormality, nausea, gastric appetite, and constipation (Table 1).
According to the viral shedding time of anal/rectal swabs and stool specimens, these cases can be divided further into five groups: less than 14 days, 14–28 days, 28–42 days, 42–56 days, and more than 56 days. In 60% of the cases, the gastrointestinal shedding time was between 28 and 42 days, while only 4% of the cases presented shedding time shorter than 14 days, and only 2% of the cases reported shedding time longer than 56 days. The number of cases in the remaining two groups accounted for 26% and 8%, respectively (Fig. 2).
Discussion
SARS-CoV-2, SARS-CoV, and the Middle East respiratory syndrome-CoV (MERS-CoV) are three new species from the same coronavirus family, which are notorious to global people as they caused epidemics of serious respiratory disease. A clear difference between them was the detection of viral RNA in stool. It was reported that MERS-CoV RNA was detectable in only 14.6% of stool samples from infected patients [28], while for SARS-CoV and SARS-CoV-2, the RNA prevalence in stool samples was very high. Ling et al. found that the SARS-CoV-2 RNA can be detected in the stool of 81.8% (54/66) adult patients, and the median time from the onset of symptoms to first negative RT-PCR results for stool specimens was 11.0 (9.0–16.0) days [29]. Chen et al. found that the median duration time of positive RT-PCR test results for viral RNA in feces was 9, 8, and 14 days in uncomplicated, mild, and severe adult patients, respectively [30]. In one systematic review of 55 studies (1348 patients), the median duration of fecal RT-PCR positivity of children and adults was 22 days and 18 days, respectively [31]. Another meta-analysis suggested a median duration of 19.2 days for fecal viral clearance in adults [32]. In this study, we investigated the duration of gastrointestinal viral shedding in children and found that 60% of the cases were between 28 and 42 days, and some children presented viral excretion time of over 56 days. These findings demonstrated that children with COVID-19 have a longer-term fecal viral shedding than adults.
The reasons why children need a longer time to shed SARS-CoV-2 in their stool have not yet been fully understood. In the following, we summarize several possible mechanisms. First, SARS-CoV-2 gains entry to cells through the ACE2 receptor [33], which has been detected in intestinal cells [34]. Recently, in vitro models of SARS-CoV-2 infection show that the pediatric and late fetal gastric organoids are susceptible to infection with SARS-CoV-2, while viral replication is significantly lower in undifferentiated organoids of adult origin [35]. The different expression of ACE2 in the intestines from children and adults may play a role in the duration of gastrointestinal viral shedding. Second, the duration of viral shedding is related closely to the host immune status. Several studies have suggested that immunocompromised COVID-19 patients may have prolonged periods of SARS-CoV-2 viral shedding[36, 37]. A study of 104 COVID-19 patients by Hao et al. reported that a decrease in T cells and B cells was associated with prolonged viral RNA shedding [38]. For other respiratory viral infections, such as influenza [39], adenovirus [40], and norovirus [41], current data available suggest high rates of asymptomatic carriage in the stool and a prolonged carrier state in children, which are related in part to children’s current stage of immune development. It can be interpreted that prolonged fecal viral RNA shedding in children with COVID-19 may be related to the immaturity of their immune systems. Third, gut microbiota has been reported to play a key role in determining the sensitivity of patients to viral infection. An animal study showed that the bacterial microbiome prevented persistent murine norovirus infection via the replenishment of the bacterial microbiota related to host immune specificity [41]. Another research has confirmed that Coprobacillus spp. has been observed to upregulate ACE2 in the murine gut [42]. Recently emerging evidence has suggested a link between the infection of COVID-19 and gut microbiome status [43–45]. Studies show that when compared to the microbiota of adults, children have less diverse microbiota despite the higher bacterial load [46], and thus, the differences in the gut microbiota may alter the ability of the virus to gain cellular entry into the gut, which may further influence the duration of viral shedding. Four, other factors could also play a role. Ma et al. [3] think that children often have poorer hand hygiene practices, causing contamination of the gastrointestinal tract through repeated touching with hands containing the virus or its fragments, and they are more prone to silent aspiration, and thus, the virus in the sputum or saliva may enter the gastrointestinal tract through swallowing.
In addition to the reasons for prolonged viral shedding in feces, the infectivity of these particles and whether they harbor the potential to be spread fecally orally have yet to be discussed. During the SARS-CoV-1 outbreak in 2003, high concentrations of SARS CoV were found in the feces and urine of a patient, which, leading to the formation of viral aerosols, and later studies suggest that the plumbing and ventilation systems interacted to transmit the SARS CoV at an apartment complex in Hong Kong rapidly [47]. Recent studies have been able to isolate live viruses from stool or rectal swabs [5, 48, 49]. Lin et al. demonstrated that gastrointestinal symptoms can be more severe when the SARS-CoV-2 is present in gastrointestinal tissue confirming by endoscopy [50]. Moreover, it has been reported that viral particles in environmental settings may remain viable for up to 3 h in aerosols and 72 h on solid surfaces [51]. These findings suggested that viral shedding in the stool could be contributing to a possible route of transmission.
Recently, a new SARS-CoV-2 variant called Omicron was first reported in South Africa and quickly spread to other countries [52]. Compared with the previous variants, Omicron appears to be more infectious but causes a milder disease with younger patients and fewer hospitalizations [53, 54]. Given the situation, mild and atypical presentations of the infection in children may make early discovery difficult, and combined with the prolonged viral shedding in their feces and poorer hand hygiene practices, it may lead to further transmission of the disease.
Conclusion
Children with COVID-19 show prolonged fecal shedding compared to adults. The poor hand hygiene practices of children, their tendency to swallow sputum and/or saliva, the significant difference in expression of ACE2 in the intestine between children and adults, and the variance in immune status and intestinal microbiome could be considered as potential casual agents of longer fecal viral shedding duration of children. Viral shedding in the stool could be contributing to a possible route of transmission. Therefore, to reduce the spread of the disease, further preventive measures in children should be taken, including hand hygiene and disinfection of public areas and health places.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- COVID-19:
-
Coronavirus disease 2019
- PCR:
-
Polymerase chain reaction
- RNA:
-
Ribonucleic acid
- SARSCoV-2:
-
Syndrome coronavirus 2
- 2019-nCoV:
-
2019 Novel coronavirus
- MERS-CoV:
-
Middle East respiratory syndrome coronavirus
References
Kelvin AA, Halperin S (2020) COVID-19 in children: the link in the transmission chain. Lancet Infect Dis 20:633–634. https://doi.org/10.1016/S1473-3099(20)30236-X
Xing Y-H, Ni W, Wu Q, Li W-J, Li G-J, Wang W-D, Tong J-N, Song X-F, Wing-Kin Wong G, Xing Q-S (2020) Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect 53:473–480. https://doi.org/10.1016/j.jmii.2020.03.021
Ma X, Su L, Zhang Y, Zhang X, Gai Z, Zhang Z (2020) Do children need a longer time to shed SARS-CoV-2 in stool than adults? J Microbiol Immunol Infect 53:373–376. https://doi.org/10.1016/j.jmii.2020.03.010
Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, Guo Q, Sun X, Zhao D, Shen J et al (2020) Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 26:502–505. https://doi.org/10.1038/s41591-020-0817-4
Xiao F, Sun J, Xu Y, Li F, Huang X, Li H, Zhao J, Huang J, Zhao J (2020) Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis 26:1920–1922. https://doi.org/10.3201/eid2608.200681
Cai J, Wang X, Zhao J, Ge Y, Xu J, Tian H, Chang H, Xia A, Wang J, Zhang J et al (2020) Comparison of clinical and epidemiological characteristics of asymptomatic and symptomatic SARS-CoV-2 infection in children. Virol Sin 35:803–810. https://doi.org/10.1007/s12250-020-00312-4
Chen S, Si J, Tang W, Zhang A, Pan L, An M, Zhang H, Xue S, Wu K, Chen S et al (2020) An asymptomatic SARS-CoV-2-infected infant with persistent fecal viral RNA shedding in a family cluster: a rare case report. Front Med (Lausanne) 7:562875–562875. https://doi.org/10.3389/fmed.2020.562875
Cho SM, Ha GY (2020) A case of COVID-19 in a 45-day-old infant with persistent fecal virus shedding for more than 12 weeks. Yonsei Med J 61:901–903. https://doi.org/10.3349/ymj.2020.61.10.901
De Ioris MA, Scarselli A, Ciofi Degli Atti ML, Ravà L, Smarrazzo A, Concato C, Romani L, Scrocca R, Geremia C, Carletti M et al (2020) Dynamic viral severe acute respiratory syndrome coronavirus 2 RNA shedding in children: preliminary data and clinical consideration from a Italian regional center. Journal of the Pediatric Infectious Diseases Society 9:366–369. https://doi.org/10.1093/jpids/piaa065
Dong Q-Q, Qiu L-R, Cheng L-M, Shu S-N, Chen Y, Zhao Y, Hao Y, Shi H, Luo X-P (2020) Kindergartens reopening in the period of regular epidemic prevention and control, benefitial or harmful? Curr Med Sci 40:817–821. https://doi.org/10.1007/s11596-020-2257-2
Fan Q, Pan Y, Wu Q, Liu S, Song X, Xie Z, Liu Y, Zhao L, Wang Z, Zhang Y et al (2020) Anal swab findings in an infant with COVID-19. Pediatr Investig 4:48–50. https://doi.org/10.1002/ped4.12186
Han MS, Seong M-W, Heo EY, Park JH, Kim N, Shin S, Cho SI, Park SS, Choi EH (2020) Sequential analysis of viral load in a neonate and her mother infected with severe acute respiratory syndrome coronavirus 2. Clin Infect Dis 71:2236–2239. https://doi.org/10.1093/cid/ciaa447
Hua C-Z, Miao Z-P, Zheng J-S, Huang Q, Sun Q-F, Lu H-P, Su F-F, Wang W-H, Huang L-P, Chen D-Q et al (2020) Epidemiological features and viral shedding in children with SARS-CoV-2 infection. J Med Virol 92:2804–2812. https://doi.org/10.1002/jmv.26180
Jiehao C, Jin X, Daojiong L, Zhi Y, Lei X, Zhenghai Q, Yuehua Z, Hua Z, Ran J, Pengcheng L et al (2020) A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis 71:1547–1551. https://doi.org/10.1093/cid/ciaa198
Kam K-Q, Yung CF, Cui L, Tzer Pin Lin R, Mak TM, Maiwald M, Li J, Chong CY, Nadua K, Tan NWH et al (2020) A well infant with coronavirus disease 2019 with high viral load. Clin Infect Dis 71:847–849. https://doi.org/10.1093/cid/ciaa201
Liu P, Cai J, Jia R, Xia S, Wang X, Cao L, Zeng M, Xu J (2020) Dynamic surveillance of SARS-CoV-2 shedding and neutralizing antibody in children with COVID-19. Emerging Microbes & Infections 9:1254–1258. https://doi.org/10.1080/22221751.2020.1772677
Park JY, Han MS, Park KU, Kim JY, Choi EH (2020) First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci 35:e124-e124. https://doi.org/10.3346/jkms.2020.35.e124
Slaats MALJ, Versteylen M, Gast KB, Oude Munnink BB, Pas SD, Bentvelsen RG, van Beek R (2020) Case report of a neonate with high viral SARSCoV-2 loads and long-term virus shedding. J Infect Public Health 13:1878–1884. https://doi.org/10.1016/j.jiph.2020.10.013
Tan Y-p, Tan B-y, Pan J, Wu J, Zeng S-z, Wei H-y (2020) Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha. China Journal of Clinical Virology 127:104353. https://doi.org/10.1016/j.jcv.2020.104353
Tang A, Tong Z-D, Wang H-L, Dai Y-X, Li K-F, Liu J-N, Wu W-J, Yuan C, Yu M-L, Li P et al (2020) Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis 26:1337–1339. https://doi.org/10.3201/eid2606.200301
Wolf GK, Glueck T, Huebner J, Muenchhoff M, Hoffmann D, French LE, Keppler OT, Protzer U (2020) Clinical and epidemiological features of a family cluster of symptomatic and asymptomatic severe acute respiratory syndrome coronavirus 2 infection. Journal of the Pediatric Infectious Diseases Society 9:362–365. https://doi.org/10.1093/jpids/piaa060
Zhang B, Liu S, Dong Y, Zhang L, Zhong Q, Zou Y, Zhang S (2020) Positive rectal swabs in young patients recovered from coronavirus disease 2019 (COVID-19). J Infect 81:e49–e52. https://doi.org/10.1016/j.jinf.2020.04.023
Zhang T, Cui X, Zhao X, Wang J, Zheng J, Zheng G, Guo W, Cai C, He S, Xu Y (2020) Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol 92:909–914. https://doi.org/10.1002/jmv.25795
Holm-Jacobsen JN, Vonasek JH, Hagstrøm S, Donneborg ML, Sørensen S (2021) Prolonged rectal shedding of SARS-CoV-2 in a 22-day-old-neonate: a case report. BMC Pediatr 21:506–506. https://doi.org/10.1186/s12887-021-02976-7
Mohanty MC, Taur PD, Sawant UP, Yadav RM, Potdar V (2021) Prolonged fecal shedding of SARS-CoV-2 in asymptomatic children with inborn errors of immunity. J Clin Immunol 41:1748–1753. https://doi.org/10.1007/s10875-021-01132-1
Tariverdi M, Farahbakhsh N, Gouklani H, Khosravifar F, Tamaddondar M (2021) Dysentery as the only presentation of COVID-19 in a child: a case report. J Med Case Rep 15:65–65. https://doi.org/10.1186/s13256-021-02672-1
Uda K, Okita K, Soneda K, Taniguchi K, Horikoshi Y (2021) Kawasaki disease following coronavirus disease 2019 with prolonged fecal viral shedding. Pediatr Int 63:597–599. https://doi.org/10.1111/ped.14452
Corman VM, Albarrak AM, Omrani AS, Albarrak MM, Farah ME, Almasri M, Muth D, Sieberg A, Meyer B, Assiri AM et al (2015) Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis 62:477–483. https://doi.org/10.1093/cid/civ951
Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, Wu F, Song ZG, Huang W, Chen J (2020) Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J 133(9):1039–1043. https://doi.org/10.1097/CM9.0000000000000774
Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L, Yang Y, Liu B, Wang W, Wei C et al (2020) The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol 92:833–840. https://doi.org/10.1002/jmv.25825
Morone G, Palomba A, Iosa M, Caporaso T, De Angelis D, Venturiero V, Savo A, Coiro P, Carbone D, Gimigliano F et al (2020) Incidence and persistence of viral shedding in COVID-19 post-acute patients with negativized pharyngeal swab: a systematic review. Front Med (Lausanne) 7:562. https://doi.org/10.3389/fmed.2020.00562
Elshazli RM, Kline A, Elgaml A, Aboutaleb MH, Salim MM, Omar M, Munshi R, Mankowski N, Hussein MH, Attia AS et al (2021) Gastroenterology manifestations and COVID-19 outcomes: a meta-analysis of 25,252 cohorts among the first and second waves. J Med Virol 93:2740–2768. https://doi.org/10.1002/jmv.26836
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271-280.e278. https://doi.org/10.1016/j.cell.2020.02.052
Hamming I, Timens W, Bulthuis MLC, Lely AT, Goor HV (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203:631–637. https://doi.org/10.1002/path.1570
Giobbe GG, Bonfante F, Jones BC, Gagliano O, Luni C, Zambaiti E, Perin S, Laterza C, Busslinger G, Stuart H et al (2021) SARS-CoV-2 infection and replication in human gastric organoids. Nat Commun 12:6610–6610. https://doi.org/10.1038/s41467-021-26762-2
Dolan SA, Mulcahy Levy J, Moss A, Pearce K, Butler M, Jung S, Dominguez SR, Mwangi E, Maloney K, Rao S (2021) SARS-CoV-2 persistence in immunocompromised children. Pediatr Blood Cancer 68:e29277–e29277. https://doi.org/10.1002/pbc.29277
El Dannan H, Al Hassani M, Ramsi M (2020) Clinical course of COVID-19 among immunocompromised children: a clinical case series. BMJ case reports 13:e237804. https://doi.org/10.1136/bcr-2020-237804
Hao S, Lian J, Lu Y, Jia H, Hu J, Yu G, Wang X, Xu K, Ni Q, Li Y et al (2020) Decreased B cells on admission associated with prolonged viral RNA shedding from the respiratory tract in coronavirus disease 2019: a case-control study. J Infect Dis 222:367–371. https://doi.org/10.1093/infdis/jiaa311
Lau LLH, Ip DKM, Nishiura H, Fang VJ, Chan K-H, Peiris JSM, Leung GM, Cowling BJ (2013) Heterogeneity in viral shedding among individuals with medically attended influenza A virus infection. J Infect Dis 207:1281–1285. https://doi.org/10.1093/infdis/jit034
Munoz RE, Piedra PA, Demmler GJ (1998) Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis 1194–1200. https://doi.org/10.1086/514978
Lin PC, Yang YSH, Lin SC, Lu MC, Tsai YT, Lu SC, Chen SH, Chen SY (2022) Clinical significance and intestinal microbiota composition in immunocompromised children with norovirus gastroenteritis. PLoS ONE 17:e0266876. https://doi.org/10.1371/journal.pone.0266876
Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D et al (2017) Mining the human gut microbiota for immunomodulatory organisms. Cell 168:928-943.e911. https://doi.org/10.1016/j.cell.2017.01.022
Yeoh YK, Zuo T, Lui GC-Y, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS et al (2021) Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70:698–706. https://doi.org/10.1136/gutjnl-2020-323020
Walton GE, Gibson GR, Hunter KA (2021) Mechanisms linking the human gut microbiome to prophylactic and treatment strategies for COVID-19. Br J Nutr 126:219–227. https://doi.org/10.1017/S0007114520003980
Al-Beltagi M, Saeed NK, Bediwy AS, El-Sawaf Y (2021) Paediatric gastrointestinal disorders in SARS-CoV-2 infection: epidemiological and clinical implications. World J Gastroenterol 27:1716–1727. https://doi.org/10.3748/wjg.v27.i16.1716
Shah V (2021) Letter to the editor: microbiota in the respiratory system-a possible explanation to age and sex variability in susceptibility to SARS-CoV-2. Microbiol Insights 14:1178636120988604–1178636120988604. https://doi.org/10.1177/1178636120988604
Mckinney KR, Gong YY, Lewis TG (2006) Environmental transmission of SARS at Amoy Gardens. J Environ Health 68(9):26–52
Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (2020) Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323:1843–1844. https://doi.org/10.1001/jama.2020.3786
Zhou J, Li C, Liu X, Chiu MC, Zhao X, Wang D, Wei Y, Lee A, Zhang AJ, Chu H et al (2020) Infection of bat and human intestinal organoids by SARS-CoV-2. Nat Med 26:1077–1083. https://doi.org/10.1038/s41591-020-0912-6
Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, Gu Z, Gao L, Shi H, Mai L et al (2020) Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 69(6):997–1001. https://doi.org/10.1136/gutjnl-2020-321013
Altamimi E (2020) Effect of COVID-19 pandemic and lockdown on children with gastrointestinal disorders. Gastroenterol Res 13(3):125–128. https://doi.org/10.14740/gr1290
Thakur V, Ratho RK (2021) OMICRON (B.1.1.529): a new SARS-CoV-2 variant of concern mounting worldwide fear. J Med Virol. https://doi.org/10.1002/jmv.27541
He X, Hong W, Pan X, Lu G (2020) Wei X (2021) SARS-CoV-2 Omicron variant: characteristics and prevention. MedComm 2:838–845. https://doi.org/10.1002/mco2.110
Maslo C, Friedland R, Toubkin M, Laubscher A, Kama B (2021) Characteristics and outcomes of hospitalized patients in south africa during the COVID-19 omicron wave compared with previous waves. JAMA, J Am Med Assoc 327(6):583–584. https://doi.org/10.1001/jama.2021.24868
Acknowledgements
The authors would like to thank the support of the Key Laboratory of Pediatric Respiratory Disease, Jinan Children’s Hospital.
Funding
This work was supported by the Key Laboratory of Pediatric Respiratory Disease (Xiang Ma is the PI).
Author information
Authors and Affiliations
Contributions
X. Ma and W.T. Li contributed to the conception and design of the manuscript. W.T. Li edited the manuscript. All the authors contributed to the literature search, revised the article, and approved its final version.
Corresponding author
Ethics declarations
Ethical approval
This is a review article. No ethical approval is required.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, WT., Zhang, Y., Liu, M. et al. Prolonged viral shedding in feces of children with COVID-19: a systematic review and synthesis of data. Eur J Pediatr 181, 4011–4017 (2022). https://doi.org/10.1007/s00431-022-04622-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04622-5