Abstract
Adolescent obesity is a serious health problem associated with many comorbidities. Obesity-related alterations in circadian rhythm have been described for nocturnal blood pressure and for metabolic functions. We believe renal circadian rhythm is also disrupted in obesity, though this has not yet been investigated. This study aimed to examine renal circadian rhythm in obese adolescents before and after weight loss.
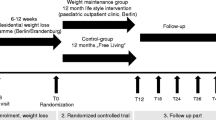
In 34 obese adolescents (median age 15.7 years) participating in a residential weight loss program, renal function profiles and blood samples were collected at baseline, after 7 months, and again after 12 months of therapy. The program consisted of dietary restriction, increased physical activity, and psychological support. The program led to a median weight loss of 24 kg and a reduction in blood pressure. Initially, lower diurnal free water clearance (− 1.08 (− 1.40–− 0.79) mL/min) was noticed compared with nocturnal values (0.75 (− 0.89–− 0.64) mL/min). After weight loss, normalization of this inverse rhythm was observed (day − 1.24 (− 1.44–1.05) mL/min and night − 0.98 (− 1.09–− 0.83) mL/min). A clear circadian rhythm in diuresis rate and in renal clearance of creatinine, solutes, sodium, and potassium was seen at all time points. Furthermore, we observed a significant increase in sodium clearance. Before weight loss, daytime sodium clearance was 0.72 mL/min (0.59–0.77) and nighttime clearance was 0.46 mL/min (0.41–0.51). After weight loss, daytime clearance increased to 0.99 mL/min (0.85–1.17) and nighttime clearance increased to 0.78 mL/min (0.64–0.93).
Conclusion: In obese adolescents, lower diurnal free water clearance was observed compared with nocturnal values. Weight loss led to a normalization of this inverse rhythm, suggesting a recovery of the anti-diuretic hormone activity. Both before and after weight loss, clear circadian rhythm of diuresis rate and renal clearance of creatinine, solutes, sodium, and potassium was observed.
What is Known: • Obesity-related alterations in circadian rhythm have been described for nocturnal blood pressure and for metabolic functions. We believe renal circadian rhythm is disrupted in obesity, though this has not been investigated yet. | |
What is New: • In obese adolescents, an inverse circadian rhythm of free water clearance was observed, with higher nighttime free water clearance compared with daytime values. Weight loss led to a normalization of this inverse rhythm, suggesting a recovery of the anti-diuretic hormone activity. • Circadian rhythm in diuresis rate and in the renal clearance of creatinine, solutes, sodium, and potassium was preserved in obese adolescents and did not change after weight loss. |




Similar content being viewed by others
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ADH:
-
Anti-diuretic hormone
- AGT:
-
Angiotensinogen
- AngII:
-
Angiotensin II
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- BSA:
-
Body surface area
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- FWC:
-
Free water clearance
- GFR:
-
Glomerular filtration rate
- RAAS:
-
Renin-angiotensin-aldosterone system
- RFP:
-
Renal function profile
- RPF:
-
Renal plasma flow
References
Braet C, Tanghe A, De Bode P, Franckx HVWM (2003) Inpatient treatment of obese children: a multicomponent programme without stringent calorie restriction. https://doi.org/10.1007/s00431-003-1155-5
Bruyndonckx L, Hoymans VY, De Guchtenaere A, Van Helvoirt M, Van Craenenbroeck EM, Frederix G, Lemmens K, Vissers DK, Vrints CJ, Ramet J, Conraads VM (2015) Diet, exercise, and endothelial function in obese adolescents. Pediatrics 135(3):e653–e661. https://doi.org/10.1542/peds.2014-1577
Cabandugama PK, Gardner MJ, Sowers JR (2017) The renin angiotensin aldosterone system in obesity and hypertension. Med Clin North Am 101(1):129–137. https://doi.org/10.1016/j.mcna.2016.08.009
Caprio S, Pierpont B, Kursawe R (2018) The “adipose tissue expandability” hypothesis: a potential mechanism for insulin resistance in obese youth. Horm Mol Biol Clin Investig 33(2). https://doi.org/10.1515/hmbci-2018-0005
Chagnac A (2003) The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14(6):1480–1486. https://doi.org/10.1097/01.ASN.0000068462.38661.89
Cooper R, McFarlane-Anderson N, Bennett F, Wilks R, Puras A, Tewksbury D, Ward R, Forrester T (1997) ACE, angiotensinogen and obesity: a potential pathway leading to hypertension. J Hum Hypertens 11(2):107–111. https://doi.org/10.1038/sj.jhh.1000391
da Silva AA, do Carmo J, Dubinion J, Hall JE (2009) The role of the sympathetic nervous system in obesity-related hypertension. Curr Hypertens Rep 11(3):206–211
Davies HO, Popplewell M, Singhal R, Smith N, Bradbury AW (2017) Obesity and lower limb venous disease - the epidemic of phlebesity. Phlebology 32(4):227–233. https://doi.org/10.1177/0268355516649333
De Guchtenaere A, Vande Walle C, Van Sintjan P, Raes A, Donckerwolcke R, Van Laecke E, Hoebeke P, Vande Walle J (2007) Nocturnal polyuria is related to absent circadian rhythm of glomerular filtration rate. J Urol 178(6):2626–2629. https://doi.org/10.1016/j.juro.2007.08.028
Dossche L, Walle JV, Van Herzeele C (2016) The pathophysiology of monosymptomatic nocturnal enuresis with special emphasis on the circadian rhythm of renal physiology. Eur J Pediatr 175(6):747–754. https://doi.org/10.1007/s00431-016-2729-3
Gómez SF, Casas Esteve R, Subirana I, Serra-Majem L, Fletas Torrent M, Homs C, Bawaked RA, Estrada L, Fíto M, Schröder H (2018) Effect of a community-based childhood obesity intervention program on changes in anthropometric variables, incidence of obesity, and lifestyle choices in Spanish children aged 8 to 10 years. Eur J Pediatr 177(10):1531–1539. https://doi.org/10.1007/s00431-018-3207-x
Greene AK, Grant FD, Slavin SA (2012) Lower-extremity lymphedema and elevated body-mass index. N Engl J Med 366(22):2136–2137. https://doi.org/10.1056/NEJMc1201684
Gulur DM, Mevcha AM, Drake MJ (2011) Nocturia as a manifestation of systemic disease. BJU Int 107(5):702–713. https://doi.org/10.1111/j.1464-410X.2010.09763.x
Hall JE, Henegar JR, Dwyer TM, Liu J, da Silva AA, Kuo JJ, Tallam L (2004) Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther 11(1):41–54. https://doi.org/10.1053/j.arrt.2003.10.007
Harp JB, Henry SA, DiGirolamo M (2002) Dietary weight loss decreases serum angiotensin-converting enzyme activity in obese adults. Obes Res 10(10):985–990. https://doi.org/10.1038/oby.2002.134
Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE (2001) Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 12(6):1211–1217
Kamperis K, Hansen M, Hagstroem S, Hvistendahl G, Djurhuus J, Rittig S (2004) The circadian rhythm of urine production, and urinary vasopressin and prostaglandin E2 exretion in healthy children. J Urol 171(6 Part 2):2571–2575. https://doi.org/10.1097/01.ju.0000110421.71910.c0
Kiba K, Hirayama A, Yishikawa M, Yutaka Y, Torimoto K, Nobutaka S, Tanaka N, Fujimoto K, Uemura H (2018) Increased urine production due to leg fluid displacement reduces hours of undisturbed sleep. LUTS Low Urin Tract Symptoms 10(3):253–258. https://doi.org/10.1111/luts.12176
Kovesdy C, Furth S, Zoccali C, World Kidney Day (2017) Obesity and kidney disease: hidden consequences of the epidemic. Indian J Nephrol 27(2):85–92. https://doi.org/10.4103/ijn.IJN_61_17
Lurbe E, Alvarez V, Liao Y, Torro I, Cremades B, Redón J, Cooper R Obesity modifies the relationship between ambulatory blood pressure and natriuresis in children. Blood Press Monit 5(5-6):275–280
Macumber IR, Weiss NS, Halbach SM, Hanevold CD, Flynn JT (2016) The association of pediatric obesity with nocturnal non-dipping on 24-hour ambulatory blood pressure monitoring. Am J Hypertens 29(5):647–652. https://doi.org/10.1093/ajh/hpv147
Marcus Y, Shefer G, Stern N (2013) Adipose tissue renin–angiotensin–aldosterone system (RAAS) and progression of insulin resistance. Mol Cell Endocrinol 378(1–2):1–14. https://doi.org/10.1016/j.mce.2012.06.021
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114(2 Suppl 4th Report):555–576
Navarro-Díaz M, Serra A, Romero R, Bonet J, Bayés B, Homs M, Pérez N, Bonal J (2006) Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol 17(12 suppl 3):S213–S217. https://doi.org/10.1681/ASN.2006080917
Noh J-Y, Han D-H, Yoon J-A, Kim M-H, Kim S-E, Ko I-G, Kim K-H, Kim C-J, Cho S (2011) Circadian rhythms in urinary functions: possible roles of circadian clocks? Int Neurourol J 15(2):64–73. https://doi.org/10.5213/inj.2011.15.2.64
Pagano ES, Spinedi E, Gagliardino JJ (2017) White adipose tissue and circadian rhythm dysfunctions in obesity: pathogenesis and available therapies. Neuroendocrinology 104(4):347–363. https://doi.org/10.1159/000453317
Rocchini AP, Katch V, Kveselis D, Moorehead C, Martin M, Lampman R, Gregory M (1989) Insulin and renal sodium retention in obese adolescents. Hypertension 14(4):367–374. https://doi.org/10.1161/01.HYP.14.4.367
Rüster C, Wolf G (2013) The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin Nephrol 33(1):44–53. https://doi.org/10.1016/j.semnephrol.2012.12.002
Sarzani R, Salvi F, Dessì-Fulgheri P, Rappelli A (2008) Renin–angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens 26(5):831–843. https://doi.org/10.1097/HJH.0b013e3282f624a0
Shatat IF, Flynn JT (2011) Relationships between renin, aldosterone, and 24-hour ambulatory blood pressure in obese adolescents. Pediatr Res 69(4):336–340. https://doi.org/10.1203/PDR.0b013e31820bd148
Simental-Mendía LE, Hernández-Ronquillo G, Gamboa-Gómez CI, Gómez-Díaz R, Rodríguez-Morán M, Guerrero-Romero F (2019) The triglycerides and glucose index is associated with elevated blood pressure in apparently healthy children and adolescents. Eur J Pediatr 178(7):1069–1074. https://doi.org/10.1007/s00431-019-03392-x
Thethi T, Kamiyama M, Kobori H (2012) The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr Hypertens Rep 14(2):160–169. https://doi.org/10.1007/s11906-012-0245-z
Whaley-Connell A, Sowers JR (2017) Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int 92(2):313–323. https://doi.org/10.1016/j.kint.2016.12.034
Wickman C, Kramer H (2013) Obesity and kidney disease: potential mechanisms. Semin Nephrol 33(1):14–22. https://doi.org/10.1016/j.semnephrol.2012.12.006
Wijnhoven TMA, van Raaij JMA, Spinelli A, Rito AI, Hovengen R, Kunesova M, Starc G, Rutter H, Sjöberg A, Petrauskiene A, O’Dwyer U, Petrova S, Farrugia Sant’Angelo V, Wauters M, Yngve A, Rubana I-M, Breda J (2013) WHO European childhood obesity surveillance initiative 2008: weight, height and body mass index in 6-9-year-old children. Pediatr Obes 8(2):79–97. https://doi.org/10.1111/j.2047-6310.2012.00090.x
Funding
Kim Pauwaert has received research grants from Ferring Pharmaceuticals.
Elke Bruneel has received research grants from Ferring Pharmaceuticals.
Johan Vande Walle has received consulting fees and travel reimbursements from Ferring Pharmaceuticals and payment for lectures from Ferring Pharmaceuticals and Astellas Pharma.
Karel Everaert has received research grants and payment for lectures all in contract with his institution from Ferring Pharmaceuticals, Medtronic and Astellas Pharma.
Author information
Authors and Affiliations
Contributions
Kim Pauwaert had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Vande Walle, Everaert, De Guchtenaere
Acquisition of data: Dejonckheere, Bruneel, Keersmaeckers, De Guchtenaere
Database work-up: Pauwaert, Dejonckheere, Bruneel, Van der Jeugt
Statistical analysis: Pauwaert, Dejonckheere, Bruneel, Keersmaeckers
Drafting of the manuscript: Pauwaert, Dejonckheere
Critical revision of the manuscript for important intellectual content: Everaert, Vande Walle, Roggeman, De Guchtenaere
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ghent University Hospital review board (EC 2015/1438). The Declaration of Helsinki was followed and conducted in accordance with the legal regulations in Belgium.
Informed consent
Informed consent was obtained from all individual participants and their parents included in the study.
Additional information
Communicated by Mario Bianchetti
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pauwaert, K., Dejonckheere, S., Bruneel, E. et al. The effect of a multidisciplinary weight loss program on renal circadian rhythm in obese adolescents. Eur J Pediatr 178, 1849–1858 (2019). https://doi.org/10.1007/s00431-019-03456-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03456-y