Abstract
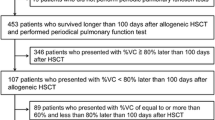
There is little data on the long-term respiratory development of children after allogeneic hematopoietic stem cell transplantation (allo-HSCT). We describe the respiratory assessment 10 years after allo-HSCT of 35 children transplanted between 2000 and 2004. During this period, 90 children were transplanted at our center. Twenty-five children died, thirty were lost to follow-up, and thirty-five came to have a pulmonary investigation. The thirty-five participants answered a questionnaire asking if they had pulmonary symptoms, and pulmonary function tests (PFTs) were performed. The median age of these children 10 years after the transplant was 16 years old. Just over a third of them had pulmonary symptoms. Among them, 5/13 (38%) had bronchiolitis obliterans syndrome (BOS). The majority of children (62.8%) did not have respiratory symptoms. PFTs were abnormal in one-third of asymptomatic children, revealing restrictive lung disease that was always mild to moderate (p = 0.02).
Conclusion: In the long term, research at the time of the medical examination for the presence of chronic cough, shortness of breath on exertion, or wheezing helps to guide the clinician as to the need for further lung exploration. Similarly, informing patients and their families about these symptoms, which can be underestimated, should allow for more specific management.
What is Known: • Pulmonary complications are a major cause of hematopoietic stem cell transplantation (HSCT) morbidity and mortality. • A long time after allogeneic HSCT, pulmonary function tests abnormalities may occur in children, but it is not always related to symptoms. | |
What is New: • The occurrence of respiratory symptoms: cough, dyspnea on exertion, chronic bronchitis, and wheezing should be systematically investigated in the follow-up of allografted patients, even at a distance. • The presence of respiratory symptoms should lead to a respiratory functional investigation to detect the presence of an obstructive syndrome. |


Similar content being viewed by others
Abbreviations
- ATS:
-
American thoracic society
- BOS:
-
Bronchiolitis obliterans syndrome
- aGvHD:
-
Acute graft-versus-host disease
- cGvHD:
-
Chronic graft-versus-host disease
- ERS:
-
European respiratory society
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Full vital capacity
- GLI:
-
Global lung function initiative
- GvHD:
-
Graft-versus-host disease
- HSCT:
-
Hematopoietic stem cell transplantation
- PFTs:
-
Pulmonary function tests
- PPFE:
-
Pleuroparenchymal fibroelastosis
- TBI:
-
Total body irradiation
- TLC:
-
Total lung capacity
References
(1995) Standardization of Spirometry, 1994 Update American Thoracic Society. Am J Respir Crit Care Med 152(3):1107–1136
Armenian SH, Sun C-L, Kawashima T, Arora M, Leisenring W, Sklar CA, Baker KS, Francisco L, Teh JB, Mills G, Wong FL, Rosenthal J, Diller LR, Hudson MM, Oeffinger KC, Forman SJ, Robison LL, Bhatia S (2011) Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 118(5):1413–1420
Au BKC, Au MA, Chien JW (2011) Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 17(7):1072–1078
Bergeron A, Chevret S, Chagnon K, Godet C, Bergot E, Peffault de Latour R, Dominique S, de Revel T, Juvin K, Maillard N, Reman O, Contentin N, Robin M, Buzyn A, Socié G, Tazi A (2015) Budesonide/formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med 191(11):1242–1249
Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, McGlave PB, Nademanee A, O’Donnell M, Ramsay NKC, Robison LL, Snyder D, Stein A, Forman SJ, Weisdorf DJ (2007) Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood 110(10):3784–3792
Chien JW (2011) Preventing and managing bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplantation. Expert Rev Respir Med 5(1):127–135
Chow EJ, Anderson L, Baker KS, Bhatia S, Guilcher GMT, Huang JT, Pelletier W, Perkins JL, Rivard LS, Schechter T, Shah AJ, Wilson KD, Wong K, Grewal SS, Armenian SH, Meacham LR, Mulrooney DA, Castellino SM (2016) Late effects surveillance recommendations among survivors of childhood hematopoietic cell transplantation: a Children’s Oncology Group report. Biol Blood Marrow Transplant 22(5):782–795
Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ (2003) Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant 9(10):657–666
Eikenberry M, Bartakova H, Defor T, Haddad IY, Ramsay NKC, Blazar BR, Milla CE, Cornfield DN (2005) Natural history of pulmonary complications in children after bone marrow transplantation. Biol Blood Marrow Transplant 11(1):56–64
Even-Or E, Ghandourah H, Ali M, Krueger J, Sweezey NB, Schechter T (2018) Efficacy of high-dose steroids for bronchiolitis obliterans syndrome post pediatric hematopoietic stem cell transplantation. Pediatr Transplant 22(2):e13155. https://doi.org/10.1111/petr.13155
Geenen MM, Cardous-Ubbink MC, Kremer LCM, van den Bos C, van der Pal HJH, Heinen RC, Jaspers MWM, Koning CCE, Oldenburger F, Langeveld NE, Hart AAM, Bakker PJM, Caron HN, van Leeuwen FE (2007) Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297(24):2705–2715
Higo H, Miyahara N, Taniguchi A, Maeda Y, Kiura K (2019) Cause of pleuroparenchymal fibroelastosis following allogeneic hematopoietic stem cell transplantation. Respir Investig 57(4):321–324
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers MED (2015) National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21(3):389–401.e1
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten C, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, ATS/ERS Task Force (2005) General considerations for lung function testing. Eur Respir J 26(1):153–161
Nishio N, Yagasaki H, Takahashi Y, Muramatsu H, Hama A, Tanaka M, Yoshida N, Watanabe N, Kudo K, Yoshimi A, Kojima S (2009) Late-onset non-infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation in children. Bone Marrow Transplant 44(5):303–308
Park M, Koh KN, Kim BE, Im HJ, Seo JJ (2011) Clinical features of late onset non-infectious pulmonary complications following pediatric allogeneic hematopoietic stem cell transplantation. Clin Transpl 25(2):E168–E176
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten C, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J (2005) Interpretative strategies for lung function tests. Eur Respir J 26(5):948–968
Prais D, Sinik MM, Stein J, Mei-Zahav M, Mussaffi H, Steuer G, Hananya S, Krauss A, Yaniv I, Blau H (2014) Effectiveness of long-term routine pulmonary function surveillance following pediatric hematopoietic stem cell transplantation. Pediatr Pulmonol 49(11):1124–1132
Pulsipher MA, Skinner R, McDonald GB et al (2012) National Cancer Institute, National Heart, Lung and Blood Institute/Pediatric Blood and Marrow Transplantation Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: the need for pediatric-specific long-term follow-up guidelines. Biol Blood Marrow Transplant 18(3):334–347
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MSM, Zheng J, Stocks J, the ERS Global Lung Function Initiative (2012) Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 40(6):1324–1343
Senn-Malashonak A, Wallek S, Schmidt K, Rosenhagen A, Vogt L, Bader P, Banzer W (2019) Psychophysical effects of an exercise therapy during pediatric stem cell transplantation: a randomized controlled trial. Bone Marrow Transplant. https://doi.org/10.1038/s41409-019-0535-z
Uhlving HH, Buchvald F, Heilmann CJ, Nielsen KG, Gormsen M, Müller KG (2012) Bronchiolitis obliterans after allo-SCT: clinical criteria and treatment options. Bone Marrow Transplant 47(8):1020–1029
Uhlving HH, Mathiesen S, Buchvald F, Green K, Heilmann C, Gustafsson P, Muller K, Nielsen KG (2015) Small airways dysfunction in long-term survivors of pediatric stem cell transplantation. Pediatr Pulmonol 50(7):704–712
Uhlving HH, Andersen CB, Christensen IJ, Gormsen M, Pedersen KD, Buchvald F, Heilmann C, Nielsen KG, Mortensen J, Moser C, Sengeløv H, Müller KG (2015) Biopsy-verified bronchiolitis obliterans and other noninfectious lung pathologies after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 21(3):531–538
Wilhelmsson M, Glosli H, Ifversen M, Abrahamsson J, Winiarski J, Jahnukainen K, Hasle H (2019) Long-term health outcomes in survivors of childhood AML treated with allogeneic HSCT: a NOPHO-AML Study. Bone Marrow Transplant 54(5):726–736
Williams KM (2017) How I treat bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Blood 129(4):448–455
Williams KM, Cheng G-S, Pusic I, Jagasia M, Burns L, Ho VT, Pidala J, Palmer J, Johnston L, Mayer S, Chien JW, Jacobsohn DA, Pavletic SZ, Martin PJ, Storer BE, Inamoto Y, Chai X, Flowers MED, Lee SJ (2016) Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant 22(4):710–716
Author information
Authors and Affiliations
Contributions
SL: collected the data and contribute to write the manuscript. KY: She is in charge of patient follow-up. CD: contribute to write the manuscript. J-HD: He is in charge of follow-up and helps the discussion. VH: in charge of lung evaluation and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
L’excellent, S., Yakouben, K., Delclaux, C. et al. Lung evaluation in 10 year survivors of pediatric allogeneic hematopoietic stem cell transplantation. Eur J Pediatr 178, 1833–1839 (2019). https://doi.org/10.1007/s00431-019-03447-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03447-z