Abstract
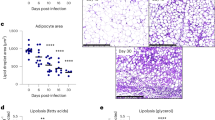
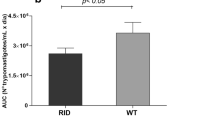
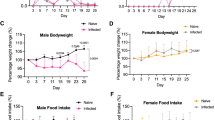
Adipose tissue is a target of Trypanosoma cruzi infection being a parasite reservoir during the chronic phase in mice and humans. Previously, we reported that acute Trypanosoma cruzi infection in mice is linked to a severe adipose tissue loss, probably triggered by inflammation, as well as by the parasite itself. Here, we evaluated how infection affects adipose tissue homeostasis, considering adipocyte anabolic and catabolic pathways, the immune–endocrine pattern and the possible repercussion upon adipogenesis. During in vivo infection, both lipolytic and lipogenic pathways are profoundly affected, since the expression of lipolytic enzymes and lipogenic enzymes was intensely downregulated. A similar pattern was observed in isolated adipocytes from infected animals and in 3T3-L1 adipocytes infected in vitro with Trypanosoma cruzi. Moreover, 3T3-L1 adipocytes exposed to plasmas derived from infected animals also tend to downregulate lipolytic enzyme expression which was less evident regarding lipogenic enzymes. Moreover, in vivo-infected adipose tissue reveals a pro-inflammatory profile, with increased leucocyte infiltration accompanied by TNF and IL-6 overexpression, and adiponectin downregulation. Strikingly, the nuclear factor PPAR-γ is strongly decreased in adipocytes during in vivo infection. Attempts to favor PPAR-γ-mediated actions in the adipose tissue of infected animals using agonists failed, indicating that inflammation or parasite-derived factors are strongly involved in PPAR-γ inhibition. Here, we report that experimental acute Trypanosoma cruzi infection disrupts both adipocyte catabolic and anabolic metabolism secondary to PPAR-γ robust downregulation, tipping the balance towards to an adverse status compatible with the adipose tissue atrophy and the acquisition of an inflammatory phenotype.






Similar content being viewed by others
References
Choe SS, Huh JY, Hwang IJ et al (2016) Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 7:30
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89:2548–2556. https://doi.org/10.1210/jc.2004-0395
Halberg N, Wernstedt-Asterholm I, Scherer PE (2008) The adipocyte as an endocrine cell. Endocrinol Metab Clin N Am 37:753–768
Scherer PE (2016) The multifaceted roles of adipose tissue—therapeutic targets for diabetes and beyond: the 2015 banting lecture. Diabetes 65:1452–1461
Luo L, Liu M (2016) Adipose tissue in control of metabolism. J Endocrinol 231:R77–R99
Yen C-LE, Stone SJ, Koliwad S et al (2008) Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49:2283–2301. https://doi.org/10.1194/jlr.R800018-JLR200
Wakil SJ (1989) Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 28:4523–4530. https://doi.org/10.1021/bi00437a001
Haemmerle G, Zimmermann R, Hayn M et al (2002) Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem 277:4806–4815. https://doi.org/10.1074/jbc.M110355200
Zimmermann R, Strauss JG, Haemmerle G et al (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386. https://doi.org/10.1126/science.1100747
Schweiger M, Schreiber R, Haemmerle G et al (2006) Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281:40236–40241. https://doi.org/10.1074/jbc.M608048200
Rosen ED, Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156:20–44
Charó NL, Rodríguez Ceschan MI, Galigniana NM et al (2016) Organization of nuclear architecture during adipocyte differentiation. Nucleus 7:249–269
Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 77:289–312. https://doi.org/10.1146/annurev.biochem.77.061307.091829
Tamori Y, Masugi J, Nishino N, Kasuga M (2002) Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 51:2045–2055. https://doi.org/10.2337/diabetes.51.7.2045
Zhang B, Berger J, Hu E et al (1996) Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol Endocrinol 10:1457–1466. https://doi.org/10.1210/mend.10.11.8923470
Imai T, Takakuwa R, Marchand S et al (2004) Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci USA 101:4543–4547. https://doi.org/10.1073/pnas.0400356101
Kim SR, Lee KS, Park HS et al (2005) Involvement of IL-10 in peroxisome proliferator-activated receptor gamma-mediated anti-inflammatory response in asthma. Mol Pharmacol 68:1568–1575. https://doi.org/10.1124/mol.105.017160
Ferreira AE, Sisti F, Sonego F et al (2014) PPAR-γ/IL-10 axis inhibits MyD88 expression and ameliorates murine polymicrobial sepsis. J Immunol 192:2357–2365. https://doi.org/10.4049/jimmunol.1302375
Choi JM, Bothwell ALM (2012) The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol Cells 33:217–222
Yang XY, Wang LH, Farrar WL (2008) A role for PPARgamma in the regulation of cytokines in immune cells and cancer. PPAR Res 2008:961753. https://doi.org/10.1155/2008/961753
Wang D, Shi L, Xin W et al (2017) Activation of PPARγ inhibits pro-inflammatory cytokines production by upregulation of miR-124 in vitro and in vivo. Biochem Biophys Res Commun 486:726–731. https://doi.org/10.1016/j.bbrc.2017.03.106
Yang XY, H WL, Chen T et al (2000) Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor γ (PPARγ) agonists. J Biol 275:4541–4545
Miettinen S, Sarkanen JR, Ashammakhi N (2008) Adipose tissue and adipocyte differentiation: molecular and cellular aspects and tissue engineering applications. Top Tissue Eng 4:1–26
Lefterova MI, Zhang Y, Steger DJ et al (2008) PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev 22:2941–2952. https://doi.org/10.1101/gad.1709008
Roggero E, Perez A, Tamae-Kakazu M et al (2002) Differential susceptibility to acute Trypanosoma cruzi infection in BALB/c and C57BL/6 mice is not associated with a distinct parasite load but cytokine abnormalities. Clin Exp Immunol 128:421–428. https://doi.org/10.1046/j.1365-2249.2002.01874.x
Roggero E, Pérez AR, Tamae-Kakazu M et al (2006) Endogenous glucocorticoids cause thymus atrophy but are protective during acute Trypanosoma cruzi infection. J Endocrinol 190:495–503. https://doi.org/10.1677/joe.1.06642
Tanowitz HB, Amole B, Hewlett D, Wittner M (1988) Trypanosoma cruzi infection in diabetic mice. Trans R Soc Trop Med Hyg 82:90–93
Manarin R, Villar SR, Bussy RF et al (2013) Reciprocal influences between leptin and glucocorticoids during acute Trypanosoma cruzi infection. Med Microbiol Immunol 202:339–352. https://doi.org/10.1007/s00430-013-0294-1
Combs TP, Nagajyothi J, Mukherjee S et al (2005) The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem 280:24085–24094. https://doi.org/10.1074/jbc.M412802200
Matos Ferreira AV, Segatto M, Menezes Z et al (2011) Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes Infect 13:1002–1005. https://doi.org/10.1016/j.micinf.2011.06.002
Nagajyothi F, Desruisseaux MS, MacHado FS et al (2012) Response of adipose tissue to early infection with Trypanosoma cruzi (Brazil Strain). J Infect Dis 205:830–840. https://doi.org/10.1093/infdis/jir840
Zingales B, Andrade SG, Briones MRS et al (2009) A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. https://doi.org/10.1590/S0074-02762009000700021
Toneatto J, Guber S, Charo NL et al (2013) Dynamic mitochondrial-nuclear redistribution of the immunophilin FKBP51 is regulated by the PKA signaling pathway to control gene expression during adipocyte differentiation. J Cell Sci 126:5357–5368. https://doi.org/10.1242/jcs.125799
Capilla J, Clemons KV, Liu M et al (2009) Saccharomyces cerevisiae as a vaccine against coccidioidomycosis. Vaccine. https://doi.org/10.1016/j.vaccine.2009.03.030
Tracey KJ, Cerami A (1990) Metabolic responses to cachectin/TNF. A brief review. Ann N Y Acad Sci 587:325–331
Maudelonde T, Lluis F (2005) PPAR gamma ligands and the control of metabolism. Med Ther Med la Reprod 7:127–132
Larsen TM, Toubro S, Astrup A (2003) PPARgamma agonists in the treatment of type II diabetes: Is increased fatness commensurate with long-term efficacy? Int J Obes 27:147–161
Krysko O, Holtappels G, Zhang N et al (2011) Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy Eur J Allergy Clin Immunol 66:396–403. https://doi.org/10.1111/j.1398-9995.2010.02498.x
Varin A, Mukhopadhyay S, Herbein G, Gordon S (2010) Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood 115:353–362. https://doi.org/10.1182/blood-2009-08-236711
Pérez AR, Tamae-Kakazu M, Pascutti MF et al (2005) Deficient control of Trypanosoma cruzi infection in C57BL/6 mice is related to a delayed specific IgG response and increased macrophage production of pro-inflammatory cytokines. Life Sci 77:1945–1959. https://doi.org/10.1016/j.lfs.2005.01.025
Zhang HH, Halbleib M, Ahmad F et al (2002) Tumor necrosis factor-α stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 51:2929–2935. https://doi.org/10.2337/diabetes.51.10.2929
Pérez AR, Roggero E, Nicora A et al (2007) Thymus atrophy during Trypanosoma cruzi infection is caused by an immuno-endocrine imbalance. Brain Behav Immun 21:890–900. https://doi.org/10.1016/j.bbi.2007.02.004
Doerrler W, Feingold KR, Grunfeld C (1994) Cytokines induce catabolic effects in cultured adipocytes by multiple mechanisms. Cytokine 6:478–484. https://doi.org/10.1016/1043-4666(94)90074-4
Feingold KR (1992) Stimulation of lipolysis in cultured fat cells by tumor necrosis factor, interleukin-1, and the interferons is blocked by inhibition of prostaglandin synthesis. Endocrinology 130:10–16. https://doi.org/10.1210/en.130.1.10
Ji C, Chen X, Gao C et al (2011) IL-6 induces lipolysis and mitochondrial dysfunction, but does not affect insulin-mediated glucose transport in 3T3-L1 adipocytes. J Bioenerg Biomembr 43:367–375. https://doi.org/10.1007/s10863-011-9361-8
Roggero E, Pérez AR, Bottasso OA et al (2009) Neuroendocrine-immunology of experimental Chagas’ disease. Ann N Y Acad Sci 1153:264–271
Roggero E, Perez AR, Pollachini N et al (2016) The sympathetic nervous system affects the susceptibility and course of Trypanosoma cruzi infection. Brain Behav Immun 58:228–236. https://doi.org/10.1016/j.bbi.2016.07.163
Duncan RE, Ahmadian M, Jaworski K et al (2007) Regulation of lipolysis in adipocytes. Annu Rev Nutr 27:79–101. https://doi.org/10.1146/annurev.nutr.27.061406.093734
Ruan H, Hacohen N, Golub TR et al (2002) Tumor necrosis factor-α suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-κB activation by TNFis obligatory. Diabetes 51:1319–1336. https://doi.org/10.2337/diabetes.51.5.1319
Jin D, Sun J, Huang J et al (2014) TNF-α reduces g0s2 expression and stimulates lipolysis through PPAR-γ inhibition in 3T3-L1 adipocytes. Cytokine 69:196–205. https://doi.org/10.1016/j.cyto.2014.06.005
Kim JY, Tillison K, Lee J-H et al (2006) The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNFin 3T3-L1 adipocytes and is a target for transactivation by PPARγ. Am J Physiol Metab 291:E115–E127. https://doi.org/10.1152/ajpendo.00317.2005
Kaufmann RL, Matson CF, Rowberg AH, Beisel WR (1976) Defective lipid disposal mechanisms during bacterial infection in rhesus monkeys. Metabolism 25:615–624. https://doi.org/10.1016/0026-0495(76)90058-5
Kaufmann RL, Matson CF, Beisel WR (1976) Hypertriglyceridemia produced by endotoxin: role of impaired triglyceride disposal mechanisms. J Infect Dis 133:548–555. https://doi.org/10.1093/infdis/133.5.548
Feingold KR, Staprans I, Memon R et al (1992) Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J Lipid Res 33:1765–1776
Lanza-jacoby S, Lansey SC, Cleary MP, Rosato FE (1982) Alterations in lipogenic enzymes and lipoprotein lipase activity during gram-negative sepsis in the rat. Arch Surg 117:144–147
Berg M, Fraker DL, Alexander HR (1994) Characterization of differentiation factor/leukaemia inhibitory factor effect of lipoprotein lipase activity and mRNA in 3T3-L1 adipocytes. Cytokine 6:425–432. https://doi.org/10.1016/1043-4666(94)90067-1
Li H, Chen X, Guan L et al (2013) MiRNA-181a regulates adipogenesis by targeting tumor necrosis factor-α (TNF-α) in the porcine model. PLoS One 8(10):e71568. https://doi.org/10.1371/journal.pone.0071568
Greenberg a S, Nordan RP, McIntosh J et al (1992) Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res 52:4113–4116
Lagathu C, Yvan-Charvet L, Bastard JP et al (2006) Long-term treatment with interleukin-1β induces insulin resistance in murine and human adipocytes. Diabetologia 49:2162–2173. https://doi.org/10.1007/s00125-006-0335-z
Oliveira A-C, Peixoto JR, de Arruda LB et al (2004) Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J Immunol 173:5688–5696. https://doi.org/10.4049/jimmunol.173.9.5688
Bafica A, Santiago HC, Goldszmid R et al (2006) Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi Infection. J Immunol 177:3515–3519. https://doi.org/10.4049/jimmunol.177.6.3515
Schaffler A, Scholmerich J (2010) Innate immunity and adipose tissue biology. Trends Immunol 31:228–235
Ajuwon KM, Banz W, Winters TA (2009) Stimulation with peptidoglycan induces interleukin 6 and TLR2 expression and a concomitant downregulation of expression of adiponectin receptors 1 and 2 in 3T3-L1 adipocytes. J Inflamm 6:8. https://doi.org/10.1186/1476-9255-6-8
Shi H, Kokoeva MV, Inouye K et al (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Investig 116:3015–3025. https://doi.org/10.1172/JCI28898.TLRs
Kopp A, Buechler C, Neumeier M et al (2009) Innate immunity and adipocyte function: ligand-specific activation of multiple toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obesity 17:648–656. https://doi.org/10.1038/oby.2008.607
Schäffler A, Schölmerich J, Salzberger B (2007) Adipose tissue as an immunological organ: toll-like receptors, C1q/TNFs and CTRPs. Trends Immunol 28:393–399
Nagajyothi F, Desruisseaux MS, Thiruvur N et al (2008) Trypanosoma cruzi Infection of cultured adipocytes results in an inflammatory phenotype. Obesity 16:1992–1997. https://doi.org/10.1038/oby.2008.331
Hammarstedt A, Andersson CX, Rotter Sopasakis V, Smith U (2005) The effect of PPARgamma ligands on the adipose tissue in insulin resistance. Prostaglandins Leukot Essent Fatty Acids 73:65–75. https://doi.org/10.1016/j.plefa.2005.04.008
Odegaard JI, Ricardo-Gonzalez RR, Goforth MH et al (2007) Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447:1116–1120. https://doi.org/10.1038/nature05894
Piccio L, Cantoni C, Henderson JG et al (2013) Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur J Immunol 43:2089–2100. https://doi.org/10.1002/eji.201242836
Guo Q, Xu L, Liu J et al (2017) Fibroblast growth factor 21 reverses suppression of adiponectin expression via inhibiting endoplasmic reticulum stress in adipose tissue of obese mice. Exp Biol Med 242:441–447. https://doi.org/10.1177/1535370216677354
Martin H (2010) Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat Res Fundam Mol Mech Mutagen 690:57–63
Rodrigues WF, Miguel CB, Chica JEL, Napimoga MH (2010) 15d-PGJ2 modulates acute immune responses to Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz 105:137–143
Hovsepian E, Mirkin GA, Penas F et al (2011) Modulation of inflammatory response and parasitism by 15-deoxy-12,14 prostaglandin J2 in Trypanosoma cruzi-infected cardiomyocytes. Int J Parasitol 41:553–562. https://doi.org/10.1016/j.ijpara.2010.12.002
Penas F, Mirkin GA, Hovsepian E et al (2013) PPARγ ligand treatment inhibits cardiac inflammatory mediators induced by infection with different lethality strains of Trypanosoma cruzi. Biochim Biophys Acta Mol Basis Dis 1832:239–248. https://doi.org/10.1016/j.bbadis.2012.08.007
Liu LF, Purushotham A, Wendel A et al (2009) Regulation of adipose triglyceride lipase by rosiglitazone. Diabetes Obes Metab 11:131–142. https://doi.org/10.1111/j.1463-1326.2008.00916.x
Festuccia WT, Blanchard P-G, Turcotte V et al (2009) Depot-specific effects of the PPARgamma agonist rosiglitazone on adipose tissue glucose uptake and metabolism. J Lipid Res 50:1185–1194. https://doi.org/10.1194/jlr.M800620-JLR200
Watkins SM, Reifsnyder PR, Pan H et al (2002) Lipid metabolome-wide effects of the PPARγ agonist rosiglitazone. J Lipid Res 43:1809–1817. https://doi.org/10.1194/jlr.M200169-JLR200
Martin G, Schoonjans K, Staels B, Auwerx J (1998) Pparγ activators improve glucose homeostasis by stimulating fatty acid uptake in the adipocytes. Atherosclerosis 137(Suppl):S75–S80
Kersten S (2001) Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep 2:282–286
Acknowledgements
ARP, GPP, JT, LD, OAB and SRV are members of The National Council Research (CONICET). FBG thanks CONICET for a fellowship. JM thanks CIN (National Interuniversity Council) for a fellowship. This work was supported by grants from the Secretary of Sciences and Technology of National University of Rosario (SCYTUNR, 1MED-348 and 1MED-372), National Agency for Scientific and Technological Promotion (ANPCYT, PICT 2013-1892) and PIP-CONICET 0614. The authors thank Wiener Lab. Foundation for their help in supplying some laboratory reagents. We thank Farré Cecilia, Esdras da Silva Oliveira Barbosa and Cabral Nicolás by their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The latest National Institute of Health guide for the care and use of laboratory was followed. The study was approved by the Institutional Animal Care and Use Committee of the School of Medicine of National University of Rosario by resolution no. 4977/2013.
Additional information
Edited by: A. Descoteaux.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
González, F.B., Villar, S.R., Toneatto, J. et al. Immune response triggered by Trypanosoma cruzi infection strikes adipose tissue homeostasis altering lipid storage, enzyme profile and adipokine expression. Med Microbiol Immunol 208, 651–666 (2019). https://doi.org/10.1007/s00430-018-0572-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-018-0572-z