Abstract
Stress evokes directed movement to escape or hide from potential danger. Corticotropin-releasing factor (CRF) neurons are highly activated by stress; however, it remains unclear how this activity participates in stress-evoked movement. The external globus pallidus (GPe) expresses high levels of the primary receptor for CRF, CRFR1, suggesting the GPe may serve as an entry point for stress-relevant information to reach basal ganglia circuits, which ultimately gate motor output. Indeed, projections from CRF neurons are present within the GPe, making direct contact with CRFR1-positive neurons. CRFR1 expression is heterogenous in the GPe; prototypic GPe neurons selectively express CRFR1, while arkypallidal neurons do not. Moreover, CRFR1-positive GPe neurons are excited by CRF via activation of CRFR1, while nearby CRFR1-negative neurons do not respond to CRF. Using monosynaptic rabies viral tracing techniques, we show that CRF neurons in the stress-activated paraventricular nucleus of the hypothalamus (PVN), central nucleus of the amygdala (CeA), and bed nucleus of the stria terminalis (BST) make synaptic connections with CRFR1-positive neurons in the GPe an unprecedented circuit connecting the limbic system with the basal ganglia. CRF neurons also make synapses on Npas1 neurons, although the majority of Npas1 neurons are arkypallidal and do not express CRFR1. Interestingly, prototypic and arkypallidal neurons receive different patterns of innervation from CRF-rich nuclei. Hypothalamic CRF neurons preferentially target prototypic neurons, while amygdalar CRF neurons preferentially target arkypallidal neurons, suggesting that these two inputs to the GPe may have different impacts on GPe output. Together, these data describe a novel neural circuit by which stress-relevant information carried by the limbic system signals in the GPe via CRF to influence motor output.





Similar content being viewed by others
References
Abdi A, Mallet N, Mohamed FY, Sharott A, Dodson PD, Nakamura KC, Suri S, Avery SV, Larvin JT, Garas FN, Garas SN, Vinciati F, Morin S, Bezard E, Baufreton J, Magill PJ (2015) Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J Neurosci 35:6667–6688
Arkadir D, Morris G, Vaadia E, Bergman H (2004) Independent coding of movement direction and reward prediction by single pallidal neurons. J Neurosci 24:10047–10056
Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, Wurthmann C, Bernstein HG, Bogerts B (1999) Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neurosci 11:71–78
Berrios GE, Wagle AC, Marková IS, Wagle SA, Rosser A, Hodges JR (2002) Psychiatric symptoms in neurologically asymptomatic Huntington’s disease gene carriers: a comparison with gene negative at risk subjects. Acta Psychiatr Scand 105:224–230
Buckley K, Kelly RB (1985) Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol 100:1284–1294
Chan RK, Brown ER, Ericsson A, Kovács KJ, Sawchenko PE (1993) A comparison of two immediate-early genes, c-fos and NGFI-B, as markers for functional activation in stress-related neuroendocrine circuitry. J Neurosci 13:5126–5138
Chan W, Costantino N, Li R, Lee SC, Su Q, Melvin D, Court DL, Liu P (2007) A recombineering based approach for high-throughput conditional knockout targeting vector construction. Nucleic Acids Res 35:e64
Chen Y, Molet J, Gunn BG, Ressler K, Baram TZ (2015) Diversity of reporter expression patterns in transgenic mouse lines targeting corticotropin-releasing hormone-expressing neurons. Endocrinology 156:4769–4780
Chometton S, Cvetkovic-Lopes V, Houdayer C, Franchi G, Mariot A, Poncet F, Fellmann D, Risold P-Y (2014) Anatomical organization of MCH connections with the pallidum and dorsal striatum in the rat. Front Syst Neurosci 8:185
Dabrowska J, Hazra R, Guo J-D, Dewitt S, Rainnie DG (2013) Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 7:156
Dissanayaka NNW, Sellbach A, Matheson S, O’Sullivan JD, Silburn PA, Byrne GJ, Marsh R, Mellick GD (2010) Anxiety disorders in Parkinson’s disease: prevalence and risk factors. Mov Disord 25:838–845
Dodson PD, Larvin JT, Duffell JM, Garas FN, Doig NM, Kessaris N, Duguid IC, Bogacz R, Butt SJB, Magill PJ (2015) Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus. Neuron 86:501–513
Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC (2007) Psychiatric symptoms in Huntington’s disease before diagnosis: the predict-HD study. Biol Psychiatry 62:1341–1346
Flandin P, Kimura S, Rubenstein JLR (2010) The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J Neurosci 30:2812–2823
Freeman SM, Walum H, Inoue K, Smith AL, Goodman MM, Bales KL, Young LJ (2014) Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus). Neuroscience 273:12–23
Füzesi T, Daviu N, Cusulin JIW, Bonin RP, Bains JS (2016) Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun 7:11937
Gittis AH, Berke JD, Bevan MD, Chan CS, Mallet N, Morrow MM, Schmidt R (2014) New roles for the external globus pallidus in basal ganglia circuits and behavior. J Neurosci 34(46):15178–15183
Glajch KE, Kelver DA, Hegeman DJ, Cui Q, Xenias HS, Augustine EC, Hernández VM, Verma N, Huang TY, Luo M, Justice NJ, Chan CS (2016) Npas1+ pallidal neurons target striatal projection neurons. J Neurosci 36:5472–5488
Grabli D (2004) Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain 127:2039–2054
Hegeman DJ, Hong ES, Hernández VM, Chan CS (2016) The external globus pallidus: progress and perspectives. Eur J Neurosci 43:1239–1265
Heinrichs SC, Koob GF (2004) Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther 311:427–440
Heinrichs SC, Menzaghi F, Merlo Pich E, Britton KT, Koob GF (1995) The role of CRF in behavioral aspects of stress. Ann N Y Acad Sci 771:92–104
Henderson R, Kurlan R, Kersun JM, Como P (1992) Preliminary examination of the comorbidity of anxiety and depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 4:257–264
Hernández VM, Hegeman DJ, Cui Q, Kelver DA, Fiske MP, Glajch KE, Pitt JE, Huang TY, Justice NJ, Chan CS (2015) Parvalbumin + neurons and Npas1 + neurons are distinct neuron classes in the mouse external globus pallidus. J Neurosci 35:11830–11847
Jackson ME, Moghaddam B (2001) Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci 21:676–681
Jiang Z, Rajamanickam S, Justice NJ (2018) Local corticotropin-releasing factor signaling in the hypothalamic paraventricular nucleus. J Neurosci 38:1874–1890
Jüngling K, Liu X, Lesting J, Coulon P, Sosulina L, Reinscheid RK, Pape H-C (2012) Activation of neuropeptide S-expressing neurons in the locus coeruleus by corticotropin-releasing factor. J Physiol 590:3701–3717
Justice NJ, Yuan ZF, Sawchenko PE, Vale W (2008) Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol 511:479–496
Kita H (2007) Globus pallidus external segment. Prog Brain Res 160:111–133. https://doi.org/10.1016/S0079-6123(06)60007-1
Kulisevsky J, Pagonabarraga J, Pascual-Sedano B, Gironell A, García-Sánchez C, Martínez-Corral M (2008) Motor changes during sertraline treatment in depressed patients with Parkinson’s disease*. Eur J Neurol 15:953–959
Lauterbach EC, Price ST, Wilson AN, Knopik VS, Jackson JG, Kavali CM (1994) Post-stroke major depression: Parkinsonism and thalamocortical systems relations. Biol Psychiatry 35:681
Lauterbach EC, Jackson JG, Price ST, Wilson AN, Kirsh AD, Dever GE (1997) Clinical, motor, and biological correlates of depressive disorders after focal subcortical lesions. J Neuropsychiatry Clin Neurosci 9:259–266
Lauterbach EC, Freeman A, Vogel RL (2003) Correlates of generalized anxiety and panic attacks in dystonia and Parkinson disease. Cogn Behav Neurol 16:225–233
Leentjens AFG, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE (2011) Symptomatology and markers of anxiety disorders in Parkinson’s disease: a cross-sectional study. Mov Disord 26:484–492
Lim MM, Murphy AZ, Young LJ (2004) Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster). J Comp Neurol 468:555–570
Liu P, Jenkins NA, Copeland NG (2003) A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13:476–484
Lowry CA, Moore FL (2006) Regulation of behavioral responses by corticotropin-releasing factor. Gen Comp Endocrinol 146:19–27
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13:133–140
Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, Nakamura KC, Magill PJ (2012) Dichotomous organization of the external globus pallidus. Neuron 74:1075–1086
Marchand WR (2010) Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Struct Funct 215:73–96
Miller JM, Vorel SR, Tranguch AJ, Kenny ET, Mazzoni P, van Gorp WG, Kleber HD (2006) Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry 163:786–788
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97
Müller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MSD, Droste SK, Kühn R, Johannes MH, Holsboer F, Wurst W (2003) Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci 6:1100–1107
Nóbrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marín O (2008) Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron 59:733–745
Nusbaum MP, Blitz DM, Marder E (2017) Functional consequences of neuropeptide and small-molecule co-transmission. Nat Rev Neurosci 18:389–403
Nuti A, Ceravolo R, Piccinni A, Dell’Agnello G, Bellini G, Gambaccini G, Rossi C, Logi C, Dell’Osso L, Bonuccelli U (2004) Psychiatric comorbidity in a population of Parkinson’s disease patients. Eur J Neurol 11:315–320
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev 20(1):128–154
Parush N, Arkadir D, Nevet A, Morris G, Tishby N, Nelken I, Bergman H (2008) Encoding by response duration in the basal ganglia. J Neurophysiol 100:3244–3252
Perkins KL (2006) Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods 154:1–18
Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M (2001) Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4:437–441
Prediger RDS, Matheus FC, Schwarzbold ML, Lima MMS, Vital MABF (2012) Anxiety in Parkinson’s disease: a critical review of experimental and clinical studies. Neuropharmacology 62:115–124
Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, Dedic N, Schumacher M, von Wolff G, Avrabos C, Touma C, Engblom D, Schutz G, Nave KA, Eder M, Wotjak CT, Sillaber I, Holsboer F, Wurst W, Deussing JM (2011) Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 333(6051):1903–1907
Saper CB (2002) The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25:433–469
Saunders A, Huang KW, Sabatini BL (2016) Globus pallidus externus neurons expressing parvalbumin interconnect the subthalamic nucleus and striatal interneurons. PLoS One 11:e0149798
Siemers ER, Shekhar A, Quaid K, Dickson H (1993) Anxiety and motor performance in Parkinson’s disease. Mov Disord 8:501–506
Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF (1998) Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20:1093–1102
Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36:165–186
Sztainberg Y, Kuperman Y, Justice N, Chen A (2011) An anxiolytic role for CRF receptor type 1 in the globus pallidus. J Neurosci 31:17416–17424
Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Kvitsani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71:995–1013
Tazi A, Swerdlow NR, LeMoal M, Rivier J, Vale W, Koob GF (1987) Behavioral activation by CRF: evidence for the involvement of the ventral forebrain. Life Sci 41:41–49
Ugolini G (1995) Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol 356:457–480
Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213:1394–1397
Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE (2000) Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428:191–212
Vine SJ, Moore LJ, Wilson MR (2016) An integrative framework of stress, attention, and visuomotor performance. Front Psychol 7:1671
Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74:858–873
Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP (1996) In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology 137:5747–5750
Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann K-K, Young JAT, Callaway EM (2007) Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53:639–647
Xue Y, Yang Y-T, Liu H-Y, Chen W-F, Chen A-Q, Sheng Q, Chen X-Y, Wang Y, Chen H, Liu H-X, Pang Y-Y, Chen L (2016) Orexin-A increases the activity of globus pallidus neurons in both normal and parkinsonian rats. Eur J Neurosci 44:2247–2257
Acknowledgements
The authors thank Z. Mao who provided expertise in confocal microscopy. We thank L. Mangieri and Q. Tong for their valuable input to data interpretation and resource sharing. We thank J. Selever, A. Herman, and B. Arenkiel for their kind gift of viral preparations necessary to perform tracing experiments. This work was supported in part by the National Institute of Neurological Disorders and Stroke Grants NS077989 to MB, NS069777 and NS047085 to CSC, MH112768 to NJJ and CSC, and MH114032 to NJJ. RD was supported by a Zilkha Family Discovery Fellowship in neuroengineering.
Author information
Authors and Affiliations
Contributions
AJH and NJJ designed experiments and analyzed the results; RD and MB designed and RD performed electrophysiological recordings. AJH, SR and ZY performed immunohistochemical and viral tracing experiments; AJH, CSC, and NJJ wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
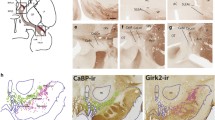
Validation of CRFR1-GFP and CRFR1-Cre transgenic mouse lines in the GPe
. In situ hybridization (ish) for iCre mRNA (A, red), with Crfr1 mRNA (B, green) shows that neurons in the GPe express both iCre and CRFR1 in the CRFR1-Cre transgenic mouse (C, arrows). Immunolocalization of GFP (D, green) and CRFR1 protein (E, red) shows that CRFR1 protein is localized to GFP+ neurons in the GPe of CRFR1-GFP mice (F, arrows). Immunolocalization of tdTomato (G) and CRFR1 protein (H) shows that tdTomato-positive GPe neurons contain CRFR1 protein in the CRFR1-Cre transgenic mouse (I, arrows). Scalebar = 50µm (EPS 14849 KB)
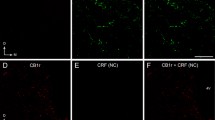
CRF is present in CRF neuron projections to the GPe
. (A) In a mouse carrying the CRFR1-GFP, CRF-Cre and lsl-tom alleles, CRFR1 GPe neurons are green fluorescent. (B) Projections from CRF neurons are red fluorescent and course through the GPe. (C) Labeling sections from these mice with CRF antibodies reveals abundant puncta in the GPe. (D) In the merged image CRF staining aligns with tomato projections, indicating that CRF is present within CRF neuron projections. We observe small puncta positive for both CRF and CRF-cre:tomato (arrows), as well as larger structures filled with CRF (arrowheads). Scalebar = 25 µm (EPS 14849 KB)
Retrograde tracing of stress-related nuclei from the GPe
. Injection of fluorescently labeled retrobeads in the GPe identifies CRF neurons in the PVN, BSTld, and CeA that project to the GPe. In CRF-Cre; lsl-L10A-GFP mice in which CRF neurons are green fluorescent (A, D, G), injections of fluorobeads in the GPe traces neurons that project to the GPe (B, E, H). The merged image of the PVN (C) shows GPe projecting neurons in red, of which 3 are also positive for CRF-cre (arrows). In the merged image of the BSTld (F), we find GPe projecting neurons in the lateral aspects of the oval subnucleus (dashed oval), of which very few are CRF positive (arrow). In the merged image of the CeA (dashed outline), we see abundant accumulation of the retrograde tracer, in which a subpopulation are also positive for CRFR1-Cre transgene expression (arrows). Scalebar = 50µm (EPS 40963 KB)
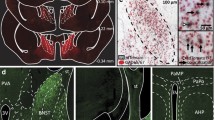
PTRV injections and quantification
. An example low power micrograph shows that Cre-dependent helper virus injections (A, AAV-G/AAV-TVA-mChy) in Npas1-Cre transgenic mice cause Npas1 neurons across broad regions of the GPe to be red fluorescent. Secondary injections of PTRV (SADΔG-GFP) infect a small number of these neurons with rabies virus (arrows, yellow in C). The rabies virus transynaptically infects neurons connected to these starter neurons (green in C). Scalebar = 100µm. (D) Location of starter neurons in quantified PTRV experiments. The left side of each atlas diagram displays the positions of starter neurons in quantified CRFR1-Cre tracing experiments (red, blue and yellow). The right side of the brain displays the position of starter neurons in quantified Npas1-Cre tracing experiments (purple, orange, green). (left) Starter neurons in rostral regions of the GPe (0 to -0.5 mm AP from bregma) collapsed onto an atlas diagram of a brain section at -0.22 mm AP from bregma. (middle) Starter neurons in the central regions of the GPe (-0.5 to -0.8 mm AP to bregma) projected onto an atlas diagram of a coronal section at -0.7 mm from bregma. (right) Starter neurons in caudal regions of the GPe (-0.8 mm to -1.7 mm AP from bregma) collapsed onto an atlas diagram of a coronal section at -1.2mm AP from bregma. (E) Quantification of starter neurons and of traced neurons in the BSTld, PVN, and CeA. Three experiments were quantified for CRFR1-cre experiments (top), and three experiments were quantified for Npas1-cre experiments (bottom) (EPS 17990 KB)
Rights and permissions
About this article
Cite this article
Hunt, A.J., Dasgupta, R., Rajamanickam, S. et al. Paraventricular hypothalamic and amygdalar CRF neurons synapse in the external globus pallidus. Brain Struct Funct 223, 2685–2698 (2018). https://doi.org/10.1007/s00429-018-1652-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1652-y