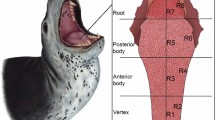
Abstract
Coordinated movement of the jaw is essential for catching and swallowing the prey. The majority of the jaw muscles in frogs are supplied by the trigeminal motoneurons. We have previously described that the primary vestibular afferent fibers, conveying information about the movements of the head, established close appositions on the motoneurons of trigeminal nerve providing one of the morphological substrates of monosynaptic sensory modulation of prey-catching behavior in the frog. The aim of our study was to reveal the spatial distribution of vestibular close appositions on the somatodendritic compartments of the functionally different trigeminal motoneurons. In common water frogs, the vestibular and trigeminal nerves were simultaneously labeled with different fluorescent dyes and the possible direct contacts between vestibular afferents and trigeminal motoneurons were identified with the help of DSD2 attached to an Andor Zyla camera. In the rhombencephalon, an overlapping area was detected between the incoming vestibular afferents and trigeminal motoneurons along the whole extent of the trigeminal motor nucleus. The vestibular axon collaterals formed large numbers of close appositions with dorsomedial and ventrolateral dendrites of trigeminal motoneurons. The majority of direct contacts were located on proximal dendritic segments closer than 300 µm to the somata. The identified contacts were evenly distributed on rostral motoneurons innervating jaw-closing muscles and motoneurons supplying jaw-opening muscles and located in the caudal part of trigeminal nucleus. We suggest that the identified contacts between vestibular axon terminals and trigeminal motoneurons may constitute one of the morphological substrates of a very quick response detected in trigeminal motoneurons during head movements.











Similar content being viewed by others
References
Anderson CW (2001) Anatomical evidence for brainstem circuits mediating feeding motor programs in the leopard frog Rana pipiens. Exp Brain Res 140:12–19
Anderson CW, Nishikawa KC (1993) A prey-type dependent hypoglossal feedback system in the frog Rana pipiens. Brain Behav Evol 42:189–196
Bácskai T, Matesz C (2002) Primary afferent fibers establish dye-coupled connections in the frog central nervous system. Brain Res Bull 57:317–319
Bácskai T, Veress G, Halasi G, Deak A, Racz E, Szekely G, Matesz C (2008) Dendrodendritic and dendrosomatic contacts between oculomotor and trochlear motoneurons of the frog, Rana esculenta. Brain Res Bull 75:419–423
Bácskai T, Veress G, Halasi G, Matesz C (2010) Crossing dendrites of the hypoglossal motoneurons: possible morphological substrate of coordinated and synchronized tongue movements of the frog, Rana esculenta. Brain Res 1313:89–96
Barbas-Henry HA, Lohman AH (1988) Primary projection and efferent cells of the VIIIth cranial nerve in the monitor lizard. Varanus exanthematicus J Comp Neurol 277:234–249
Bass AH, Marchaterre MA, Baker R (1994) Vocal–acoustic pathways in a teleost fish. J Neurosci 14:4025–4039
Bennett MVL (2000) Electrical synapses, a personal perspective (or history). Brain Res Rev32:16–18
Birinyi A, Straka H, Matesz C, Dieringer N (2001) Location of dye-coupled second order and of efferent vestibular neurons labeled from individual semicircular canal or otolith organs in the frog. Brain Res 921:44–59
Birinyi A, Kecskes S, Kovalecz G, Matesz C (2016) Termination of vestibular afferent fibers on the trigeminal motoneurons: possible network mediating jaw movements during prey-catching behavior of the frog. In: Abstract Book of 8th European Conference on Comparative Neurobiology Munich Germany, P2–02
Buisseret-Delmas C, Compoint C, Delfini C, Buisseret P (1999) Organization of reciprocal connections between trigeminal and vestibular nuclei in the rat. J Comp Neurol 409:153–168
Corbacho F, Nishikawa KC, Weerasuriya A, Liaw JS, Arbib MA (2005) Schema-based learning of adaptable and flexible prey-cathing in anurans. I. The basic architecture. Biol Cybern Neurol 93:391–409
Corson AJ, Erisir A (2013) Monosynaptic convergence of chorda tympani and glossopharyngeal afferents onto ascending relay neurons in the nucleus of the solitary tract: a high-resolution confocal and correlative electron microscopy approach. J Comp Neurol 521:2907–2926
Cuccurazzu B, Deriu F, Tolu E, Yates BJ, Billing I (2007) A monosynaptic pathway links the vestibular nuclei and masseter muscle motoneurons in rats. Exp Brain Res 176:665–671
Deriu F, Eusebio T, Rothwell JC (2005) A sound-evoked vestibulomasseteric reflex in healthy humans. J Neurophysiol 93:2739–2751
Diagne M, Valla J, Delfini C, Buisseret-Delmas C, Buisseret P (2006) Trigeminovestibular and trigeminospinal pathway in rats: retrograd tracing compared with glutamic acid decarboxylase and glutamate immunohistochemistry. J Comp Neurol 496:759–772
Dicke U, Roth G (1993) Tectosipnal pathways in plethodontoid salamanders and their connections to motor nuclei involved in prey capture. In: Elsner N, Heisenberg M (eds) Gene brain behaviour. M. Georg Thieme, Stuttgart, pp 1–92
Dieringer N (1995) Vestibular compensation: neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol 46:97–129
Ewert JP (1984) Tectal mechanism that underlies prey-catching and avoidance behavior in toads. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum, New York, pp 247–416
Ewert JP, Buxbaum-Conradi H, Glagow C, Röttgen A, Schürg-Pfeiffer E, Schwippert WW (1999) Forebrain and midbrain structures involved in prey-catching behaviour of toads: stimulus-response mediating circuits and their modulating loops. Eur J Morphol 37:172–176
Ewert JP, Buxbaum-Conradi H, Dreisvogt M, Glagow C, Merkel-Harff C, Röttgen A, Schürg-Pfeiffer E, Schwippert WW (2001) Neural modulation of visuomotor functions underlying prey-catching behaviour in anurans: perception, attention, motor performance, learning. Comp Bichem Physiol Part A 128:417–461
Gans C, Gorniak GC (1982) Functional morphology of lingual protrusion in marine toads (Bufo marinus). Am J Anat 163:195–222
Gaupp E (1904) Ecker’s und R. Wiedersheim’s Anatomie des Frosches. vol 3. Vieweg und Sohn, Braunschweig
Giaconi E, Deriu F, Tolu E, Cuccurazzu B, Yates BJ, Billing I (2006) Transneuronal tracing of vestibulo-trigeminal pathways innervating the masseter muscle in the rat. Exp Brain Res 171:330–339
Gray LA, O’Reilly JC, Nishikawa KC (1997) Evolution of forelimb movement patterns for prey manipulation in anurans. J Exp Zool 277:417–424
Grinnell AD (1966) A study of the interaction between motoneurons in the frog spinal cord. J Physiol 182:612–648
Heiss E, Natchev N, Gumpenberger M, Weissenbacher A, Van Wassenbergh S (2013) Biomechanics and hydrodynamics of prey capture in the chinese giant salamander reveal a high-performance jaw-powered suction feeding mechanism. J R Soc Interface. https://doi.org/10.1098/rsif.2012.1028
Hickenbottom RS, Bishop B, Moriarty TM (1985) Effects of whole-body rotation on masseteric motoneuron excitability. Exp Neurol 89:442–453
Hillman DE (1969) Light and electron microscopical study of the relationships between the cerbellum and the vestibular organ of the frog. Exp Brain Res 9:1–15
Hiscock J, Straznicky C (1982) Peripheral and central terminations of axons of the mesencephalic trigeminal neurons in Xenopus. Neurosci Lett 32:235–240
Horowitz SS, Simmons AM (2010) Development of tectal connectivity across metamorphosis in the bullfrog (Rana catesbeiana). Brain. Behav Evol 76:226–247
Kecskes S, Matesz C, Gaál B, Birinyi A (2016) Neural circuits underlying tongue movements for the prey-catching behavior in frog: distribution of primary afferent terminals on motoneurons supplying the tongue. Brain Struct Funct 221:1533–1553
Kottick A, Mufaddal I, Baghdadwala I, Ferguson EV, Wilson RJA (2013) Transmission of the respiratory rhytm to trigeminal and hypoglossal motor neurons in the American Bullfrog (Lhitobates catesbiana). Resp Physiol Neurobiol 188:180–191
Koyama H, Kishida R, Goris RC, Kusunoki T (1990) Organization of the primary projections of the lateral line nerves in the lamprey Lampetra japonica. J Comp Neurol 295:277–289
Kuruvilla A, Sitko S, Schwartz IR, Honrubia V (1985) Central projections of primary vestibular fibers in the bullfrog: I. The vestibular nuclei. Laryngoscope 5:692–707
Lazar G, Toth P, Csank G, Kicliter E (1983) Morphology and location of tectal projection neurons in frogs: a study with HRP and cobalt-filling. J Comp Neurol 215:108–120
Li YQ, Takada M, Kaneko T, Mizuno N (1995) Premotor neurons for trigeminal motor nucleus neurons innervating the jaw-closing and jaw-opening muscles: differential distribution in the lower brainstemin the rat. J Comp Neurol 356:563–579
Mandal R, Anderson CW (2010) Identification of muscle spindles in the submentalis muscle of the marine toad, Bufo marinus and its potential proprioceptive capacity in jaw-tongue coordination. Anat Rec (Hoboken) 293:1568–1573
Matesz C (1979) Central projection of the VIIIth cranial nerve in the frog. Neurosci 4:2061–2071
Matesz C (1988) Fine structure of the primary afferent vestibulocochlear terminals in the frog. Acta Biol Hung 39:267–277
Matesz C (1994) Synaptic relations of the trigeminal motoneurons in a frog (Rana esculenta). Eur J Morphol 32:117–121
Matesz C, Székely G (1978) The motor column and sensory projections of the branchial cranial nerves in the frog. J Comp Neurol 178:157–1769
Matesz C, Birinyi A, Hevessy Z (1994) Motoneurons differ in size and peripheral target in the trigeminal and facial nuclear complex of the frog. J Hirnforsch 35:67–70
Matesz C, Birinyi A, Kothalawala DS, Székely G (1995) Investigation of the dendritic geometry of brainstem motoneurons with different functions using multivariant multistatistical techniques in the frog. Neurosci 65:1129–1144
Matesz C, Kulik A, Bácskai T (2002) Ascending and descending projections of the lateral vestibular nucleus in the frog, Rana esculenta. J Comp Neurol 444:115–128
Matesz C, Kovalecz G, Veress G, Deák Á, Rácz É, Bácskai T (2008) Vestibulotrigeminal pathways in the frog, Rana esculenta. Brain Res Bull 75:371–374
Montgomery NM (1988) Projections of the vestibular and cerebellar nuclei in Rana pipiens. Brain Behav Evol 31:82–95
Munoz AM, Gonzalez A, ten Donkelaar HJ (1995) Anuran dorsal column nucleus: organization, immunohistochemical characterization and fiber connections in Rana perezi and Xenopus laevis. J Comp Neurol 363:197–220
Nishikawa KC, Gans C (1992) The role of hypoglossal sensory feedback during feeding in the marine toad, Bufo marinus. J Exp Biol 264:2511–2529
Olsson K, Westberg KG (1991) Integration in trigeminal premotor interneurones in the cat. 2. Functional characteristics of neurones in the subnucleus of the oral nucleus of the spinal trigeminal tract with a projection to the digastric motoneurone subnucleus. Exp Brain Res 84:115–124
Opdam R, Kemali M, Nieuwenhuys R (1976) Topological analysis of the brain stem of the frogs Rana esculenta and Rana catesbeiana. J Comp Neurol 165:307–332
Pratt KG, Aizenman CD (2009) Multisensory integration in mesencephalic trigeminal neurons in Xenopus tadpoles. J Neurophysiol 102:399–412
Precht W, Llinas R (1969) Functional organization of the vestibular afferents to the cerebellar cortex of frog and cat. Exp Brain Res 9:30–52
Precht W, Richter A, Ozawa S, Shimazu H (1974) Intracellular study of frog’s vestibular neurons in relation to the labyrinth and spinal cord. Expl Brain Res 19:377–393
Rácz E, Bácskai T, Halasi G, Kovács E, Matesz C (2006) Organization of dye-coupled cerebellar granule cells labeled from afferent vestibular and dorsal root fibers in the frog Rana esculenta. J Comp Neurol 496:382–394
Satoh Y, Ishizuka K, Murakami T (2009a) Modulation of the jaw-opening reflex by stimulation of vestibular nuclear complex in rats. Neurosci Lett 457:21–26
Satoh Y, Ishizuka K, Murakami T (2009b) Modulation of the masseteric monosynaptic reflex by stimulation of vestibular nuclear complex in rats. Neurosci Lett 466:16–20
Satoh Y, Ishizuka K, Murakami T (2010) Modulation of cortically induced rhythmic jaw movements in rats by stimulation of the vestibular nuclear complex. Neurosci Res 68:307–14
Sotello C (1976) Morphology of cerebellar cortex. In: Llinás R, Precht W (eds) Frog neurobiology. Springer, Berlin, pp 865–891
Sterio DC (1984) The unbiased estimation of number and size of arbitrary particles using the dissector. J Misrosc 134:127–136
Székely G, Matesz C (1987) Trigeminal motoneurons with disparate dendritic geometry innervate different muscle proup in the frog. Neurosci Lett 77:161–165
Székely G, Matesz C (1993) The efferent system of cranial nerve nuclei: a comparative neuromorphological study. Adv Anat Embryol Cell Biol 128:1–92
Tolu E, Caria MA, Simula ME, Lacana P (1994) Muscle spindle and periodontal trigeminal afferents modulate the hypoglossal motoneuronal activity. Arch Ital Biol 132:93–104
Tolu E, Caria MA, Chessa G, Melis F, Simula ME, Podda MV, Solinas A, Deriu F (1996) Trigeminal motoneuron responses to vestibular stimulation in Guinea pig. Arch Ital Biol 134:140–51
Valdez CM, Nishikawa KC (1997) Sensory modulation and behavioral choice during feeding in the Australian frog, Cyclorana novaehollandiae. J Comp Physiol A 180:187–202
Valla J, Delfini C, Diagne M, Pinganaud G, Buisseret P, Buisseret-Delmas C (2003) Vestibulotrigeminal and vestibulospinal projection in rats: retrograde tracing coupled to glutamic acid decarboxylase immunoreactivity. Neurosci Lett 340:225–228
Vaney DI (1991) Many diverse types of retinal neurons show tracer coupling when injected with biocytin or neurobiotin. Neurosci Lett 125:187–190
Walkowiak W (2007) Call production and neural basis of vocalization. In: Narins PM, Feng AS, Richard RF, Popper AN (eds) Hearing and sound communication in Amphibians. Springer, Berlin, pp 87–112
Westberg KG, Sandström G, Olsson K (1995) Integration in trigeminal premotor interneurones in the cat. 3. Input characteristics and synaptic actions of neurones in subnucleus of the oral nucleus of the spinal trigeminal tract with a projection to the masseteric motoneuron subnucleus. Exp Brain Res 104:449–461
Wouterlood FG, van Haeften T, Blijleven N, Perez-Templado P, Perez-Templado H (2002) Double-label confocal laser-scanning microscopy, image restoration, and real-time three-dimensional reconstruction to study axons in the central nervous system and their contacts with target neurons. Appl Immunohistochem Mol Morphol 10:85–95
Wouterlood FG, Bockers T, Witter MP (2003) Synaptic contacts between identified neurons visualized in the confocal laser scanning microscope. Neuroanatomical tracing combined with immunofluorescence detection of post-synaptic density proteins and target neuron-markers. J Neurosci Methods 128:129–142
Acknowledgements
The authors thank Ms Timea Horvath for skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare no conflict of interest.
Grant sponsor
This research was supported by financial aid from the Hungarian Academy of Sciences (MTA-TKI 11,008) and from the Hungarian National Research Found (OTKA K115471).
Rights and permissions
About this article
Cite this article
Birinyi, A., Rácz, N., Kecskes, S. et al. Neural circuits underlying jaw movements for the prey-catching behavior in frog: distribution of vestibular afferent terminals on motoneurons supplying the jaw. Brain Struct Funct 223, 1683–1696 (2018). https://doi.org/10.1007/s00429-017-1581-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1581-1