Abstract
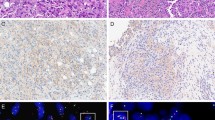
In 2018, the American Society of Clinical Oncology/College of American Pathologists revised the criteria for HER2 immunohistochemistry (IHC) equivocal (2+) classification in their updated guideline. We reviewed invasive breast cancer specimens originally classified as equivocal (2+) under the 2018 guideline that underwent HER2 fluorescence in situ hybridization (FISH) testing from August 2018 to August 2019 at our Canadian reference hospital to investigate cases with ambiguous staining patterns between the 1+ and 2+ definitions. Demographics, pathologic features, and pre-analytic conditions were recorded. The H&E and corresponding HER2 IHC slides were reviewed to confirm tumor type and grade, and classify as HER2 indeterminate, 0, 1+, 2+, or “Intermediate” (staining features between the 1+ and 2+ classifications). FISH testing was performed on 289 cases and 273 met inclusion criteria. The FISH-amplified rate was 12.1%. Upon IHC review, 44.7% (122/273) of cases were reclassified as Intermediate. These cases had incomplete staining with moderate intensity (43/122, 35.3%) and/or <10% complete weak or moderate staining (102/122, 83.6%). Intermediate cases had a significantly lower frequency of amplified FISH results than 2+ cases (p < 0.0001), with only four (3.3%) FISH positive and two (1.6%) FISH heterogeneous. Our study highlights the ambiguity in the current guideline for classifying some HER2 IHC patterns. As the rate of gene amplification in these cases was low (4.9%), we recommend adhering to the 2018 HER2 2+ criteria for reflex FISH testing. However, cases with <10% moderate complete staining and certain heterogeneous patterns warrant special consideration. Further descriptive clarification of 1+ criteria is needed.




Similar content being viewed by others
Data Availability
Will be available upon request
References
Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4784):177–182
Slamon DJ, Godolphin W, Jones LA, Holt J, Wong S, Keith D, Levin W, Stuart S, Udove J, Ullrich A (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684
Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, Winer EP, Colon-Otero G, Davidson NE, Mamounas E, Zujewski JA, Wolmark N (2014) Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32(33):3744–3752
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J, Breast Cancer International Research Group (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283
Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G Jr, Untch M, Smith I, Gianni L, Baselga J, al-Sakaff N, Lauer S, McFadden E, Leyland-Jones B, Bell R, Dowsett M, Jackisch C (2017) 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389(10075):1195–1205
Fehrenbacher L, Cecchini RS, Geyer CE et al (2020) NSABP B-47/NRG Oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol 38(5):444–453
Wolff AC, Hammond MEH, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145
Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JMS, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2014) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 138(2):241–256
Wolff AC, McShane LM, Hammond MEH et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med 142(11):1364–1382
Middleton L, Price K, Puig P et al (2009) Implementation of American Society of Clinical Oncology/College of American Pathologists HER2 guideline recommendations in a tertiary care facility increases HER2 immunohistochemistry and fluorescence in situ hybridization concordance and decreases the number of inconclusive cases. Arch Pathol Lab Med 133(5):775–780
Press MF, Sauter G, Buyse M, Fourmanoir H, Quinaux E, Tsao-Wei DD, Eiermann W, Robert N, Pienkowski T, Crown J, Martin M, Valero V, Mackey JR, Bee V, Ma Y, Villalobos I, Campeau A, Mirlacher M, Lindsay MA, Slamon DJ (2016) HER2 gene amplification testing by fluorescent in situ hybridization (FISH): comparison of the ASCO-College of American Pathologists guidelines with FISH scores used for enrollment in Breast Cancer International Research Group clinical trials. J Clin Oncol 34(29):3518–3528
Hanna WM, Slodkowska E, Lu F et al (2017) Comparative analysis of human epidermal growth factor receptor 2 testing in breast cancer according to 2007 and 2013 American Society of Clinical Oncology/College of American Pathologists guideline recommendations. J Clin Oncol 35(26):3039–3045
Rakha EA, Pigera M, Shaaban A, Shin SJ, D'Alfonso T, Ellis IO, Lee AHS (2015) National guidelines and level of evidence: comments on some of the new recommendations in the American Society of Clinical Oncology and the College of American Pathologists human epidermal growth factor receptor 2 guidelines for breast cancer. J Clin Oncol 33(11):1301–1302
Vingiani A, Maisonneuve P, Dell’Orto P et al (2013) The clinical relevance of micropapillary carcinoma of the breast: a case-control study. Histopathology 63(2):217–224
Press MF, Villalobos I, Santiago A, Guzman R, Cervantes M, Gasparyan A, Campeau A, Ma Y, Tsao-Wei DD, Groshen S (2016) Assessing the new American Society of Clinical Oncology/College of American Pathologists guidelines for HER2 testing by fluorescence in situ hybridization: experience of an academic consultation practice. Arch Pathol Lab Med 140(11):1250–1258
Bates D, Maechler M, Bolker B et al (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Yang L, Zhang Z, Li J, Chen M, Yang J, Fu J, Bu H, Tang S, Liu Y, Li H, Li X, Xu F, Teng X, Yang Y, Ma Y, Guo S, Wang J, Guo D (2018) A decision tree-based prediction model for fluorescence in situ hybridization HER2 gene status in HER2 immunohistochemistry-2+ breast cancers: a 2538-case multicenter study on consecutive surgical specimens. J Cancer 9(13):2327–2333
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, Perou CM, Regan MM, Rimm DL, Symmans WF, Torlakovic EE, Varella L, Viale G, Weisberg TF, McShane LM, Wolff AC (2020) Estrogen and progesterone receptor testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists guideline update. Arch Pathol Lab Med 144:545–564
Cheung CC, Barnes P, Bigras G, Boerner S, Butany J, Calabrese F, Couture C, Deschenes J, el-Zimaity H, Fischer G, Fiset PO, Garratt J, Geldenhuys L, Gilks CB, Ilie M, Ionescu D, Lim HJ, Manning L, Mansoor A, Riddell R, Ross C, Roy-Chowdhuri S, Spatz A, Swanson PE, Tron VA, Tsao MS, Wang H, Xu Z, Torlakovic EE (2019) Fit-for-purpose PD-L1 biomarker testing for patient selection in immuno-oncology: guidelines for clinical laboratories from the Canadian Association of Pathologists-Association Canadienne des Pathologistes (CAP-ACP). Appl Immunohistochem Mol Morphol 27(10):699–714
Onguru O, Zhang PJ (2016) The relation between percentage of immunostained cells and amplification status in breast cancers with equivocal result for Her2 immunohistochemistry. Pathol Res Pract 212(5):381–384
Efared B, Sidibé IS, Gamrani S, el Otmani I, Erregad F, Hammas N, Bennis S, Chbani L, el Fatemi H (2018) The assessment of HER2 gene status by fluorescence in situ hybridization in invasive breast carcinomas with equivocal HER2 immunostaining: experience from a single institution. Int J Surg Pathol 26(7):593–599
Gordian-Arroyo AM, Zynger DL, Tozbikian GH (2019) Impact of the 2018 ASCO/CAP HER2 guideline focused update. Am J Clin Pathol 152(1):17–26
Hariri N, Zare S, Murphy J, et al (2020) Cost-effectiveness of a dual (immunohistochemistry and fluorescence in situ hybridization) HER2/neu testing strategy on invasive breast cancers [published online ahead of print, doi:10.1097/PAI.0000000000000849]. Appl Immunohistochem Mol Morphol. 2020.
Woo JW, Lee K, Chung YR et al (2020) The updated 2018 American Society of Clinical Oncology/College of American Pathologists guideline on human epidermal growth factor receptor 2 interpretation in breast cancer: comparison with previous guidelines and clinical significance of the proposed in situ hybridization groups. Hum Pathol 98:10–21
Hanna WM, Barnes PJ, Chang MC, Gilks CB, Magliocco AM, Rees H, Quenneville L, Robertson SJ, SenGupta SK, Nofech-Mozes S (2014) Human epidermal growth factor receptor 2 testing in primary breast cancer in the era of standardized testing: a Canadian prospective study. J Clin Oncol 32(35):3967–3973
Terry J, Torlakovic EE, Garratt J, Miller D, Köbel M, Cooper J, Bahzad S, Pilavdzic D, OʼMalley F, OʼBrien AE, SenGupta S, Alport E, Têtu B, Knight B, Pettigrew NM, Berendt R, Wolber R, Trotter MJ, Riddell RH, Gaboury L, Elms F, Magliocco A, Barnes P, Gown AM, Gilks CB (2009) Implementation of a Canadian external quality assurance program for breast cancer biomarkers: an initiative of Canadian Quality Control in Immunohistochemistry (cIQc) and Canadian Association of Pathologists (CAP) national standards committee/immunohistoc. Appl Immunohistochem Mol Morphol 17(5):375–382
Marchio C, Annaratone L, Marques A et al (2020) Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol S1044-579X(20)30049-3.
Dekker TJ (2020) HER2-targeted therapies in HER2-low expressing breast cancer. J Clin Oncol 38(28):3350–3351
Modi S, Park H, Murthy R et al (2020) Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase 1b study. J Clin Oncol 28(17):1887–1896
Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, Marra A, Viale G, Trapani D, Cardoso F, Penault-Llorca F, Viale G, Andrè F, Curigliano G (2020) HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 38(17):1951–1963
Bahreini F, Soltanian AR, Mehdipour P (2015) A meta-analysis on concordance between immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to detect HER2 gene overexpression in breast cancer. Breast Cancer 22(6):615–625
Acknowledgments
We would like to acknowledge Mr. S. Whitefield of the Dalhousie University Cellular and Molecular Digital Imaging Facility for his assistance in taking and formatting photomicrographs.
Author information
Authors and Affiliations
Contributions
V. Taylor, P. Barnes, and G. Bethune contributed to data collection, analysis, and manuscript writing/editing. S. Godwin contributed to figures, statistical analysis, and manuscript writing/editing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
This study was approved by the institutions’ Research Ethics Board.
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper has not been published online or in print, and is not under consideration elsewhere. All writing is original.
This article is part of the Topical Collection on Quality in Pathology
Rights and permissions
About this article
Cite this article
Taylor, V.J., Barnes, P.J., Godwin, S.C. et al. Assessment of HER2 using the 2018 ASCO/CAP guideline update for invasive breast cancer: a critical look at cases classified as HER2 2+ by immunohistochemistry. Virchows Arch 479, 23–31 (2021). https://doi.org/10.1007/s00428-021-03034-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03034-4