Abstract
Tumor-associated macrophages (TAMs) have recently been reported as an important factor in tumor growth and the progression of cancer. The prognostic significance of localizations and densities of TAMs in triple negative cancer (TNC) of the breast is not well understood. The aim of this study was to assess the localizations and densities of the TAMs subtype in TNC and examine their clinicopathological features. The study was based on 107 TNC cases operated on at Dokkyo Medical University Hospital using the pan-macrophage marker CD68 and the M2 macrophage marker CD163 in the tumor stroma (TS) and tumor nest (TN), respectively, and examined the clinicopathological significance. Multivariate Cox regression analyses revealed that age and CD163+ TAMs in both the TS and TN were independent prognostic factors for relapse-free survival and overall survival. No correlation was found between the number of CD68+ cells or the CD163/CD68 ratio either in TS or TN, or clinicopathological features. Our study found that infiltration of CD163+ TAMs, rather than CD68+, in both TS and TN was associated with poor prognosis in TNC patients by multivariate analysis. This suggests that CD163+ TAMs may affect the prognosis of TNC by not only regulating the immune reaction by TAMs in TS, but also because of their direct influence on TN.
Similar content being viewed by others
Introduction
Tumor-associated macrophages (TAMs) have recently been reported as an important factor in tumor growth and the progression of cancer. Recently, two processes were proposed for TAMs activation: Classically-activated type 1 (M1-like) macrophages and alternatively-activated type 2 (M2-like) macrophages. M1-like macrophages, characterized by CD68 expression, produce free radicals that can lead to DNA damage with the potential to contribute to tumoricidal activity [1]. In contrast, M2-like macrophages, characterized by both CD68 and CD163 expression, are considered to promote tumor growth and metastasis by releasing chemokines, which are inflammatory growth factors [2, 3]. Previous studies confirmed that TAMs are associated with cancer survival in several organs such as hepatoma [4], gastric cancer [5], and lung cancer [6]. In breast cancer, several studies have demonstrated that TAMs are related to hormonal status, stage, lymph node (LN) status, and prognosis [7,8,9,10]. Therefore, TAMs in different regions and at different densities may have different prognostic value in breast cancer. In general, triple-negative cancer (TNC) is characterized by a lack of expression of the estrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor 2 (HER2) protein; this type is well known to have a poor prognosis [11, 12]. However, we should note that TNCs do not always correlate with poor prognosis. Therefore, to confirm the association of TAMs and TNC, a larger cohort using different statistical methods should be evaluated. Moreover, the prognostic significance of localizations and densities of CD68+ and CD163+ TAMs in TNC is not well understood. The aim of this study was to assess the localizations and densities of the macrophage markers CD68+ and CD163+ TAMs in TNC and examine their clinicopathological features.
Materials and methods
Patients
The study was based on 107 TNC cases operated on at Dokkyo Medical University Hospital between 2006 and 2018. Patient and tumor characteristics, including patient age at the time of diagnosis, tumor size, histologic grade, LN status, and follow-up data, were determined from patients’ medical records and pathology reports. Relapse-free survival (RFS) was defined as the number of months from surgical resection to the development of documented relapse, including recurrence or distant metastasis. Overall survival (OS) was recorded from the date of curative surgery to the date of breast cancer-specific death.
The present study was approved by the Ethics Committee of Dokkyo Medical University (Tochigi, Japan; registration number: 28009) and was conducted according to the Declaration of Helsinki.
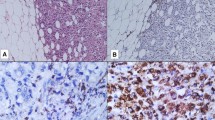
Immunohistochemistry (IHC)
Surgical sections were immunostained for ER (clone SP1, Novocastra (Leica), prediluted, nuclear), PgR (clone 1E2, Novocastra (Leica), prediluted, nuclear), HER2 (clone 4B5, Roche (VENTANA), prediluted, membranous), CD68 (CD68, clone PG-M1, Dako (Agilent), 1:50), and CD163 (CD163, clone 10D6, Novocastra (Leica), 1:50). Counterstaining was performed with hematoxylin. The percentages of nuclei stained for ER and PgR were calculated, as stated by the guideline, and a patient was considered to be “positive” if the breast tumor contained at least 1% positive cells [13]. HER2 status was assessed according to the guidelines defined by the American Society of Clinical Oncology/College of American Pathologists [14]. We estimated the TILs on hematoxylin and eosin (H&E) stained sections according to the criteria proposed by the International Immuno-Oncology Biomarkers Working Group [15]. TILs levels were categorized as high (≥ 30%) and low (< 30%) adopting previously validated cut-offs [16].
TAMs were evaluated by adapting the previously reported hotspot quantitative method [7, 10, 17,18,19]. The CD68+ and CD163+ staining was assessed by counting the number of positive macrophages. TAMs were scored as the infiltration density of CD68+ or CD163+ cells with a macrophage morphology that showed strong membranous or cytoplasmic staining. Each specimen was screened at low magnification (× 100), and the five areas with the greatest number of positively stained cells (hot spot area) were selected for further analysis. The mean macrophage count in these areas for each case was estimated at high power (× 400) magnification. The CD68+ and CD163+ macrophages were counted in the tumor stroma (TS) and tumor nest (TN) separately. The definition of TS in this study was the stromal tissue surrounding the tumor nest. TAMs in TN were defined as intraepithelial tumor-infiltrating macrophages. For statistical analyses, the number of positive cells was divided into lower and higher groups based on cut-off points according to the median. As a result, the cut-off for CD68 in TS was 26.2, CD68 in TN was 11.2, CD163 in TS was 26.6, CD163 in TN was 8.6 CD163/CD68 in TS was 1.0, CD163/CD68 in TN was 0.99 (Table 1). Two pathologists (TJ and HK) did the evaluations without access to any clinical information.
Statistical analysis
Spearman’s Rho and χ2 tests were used to compare CD68 and CD163 expression and patient and tumor characteristics. Kaplan-Meier analysis and log-rank tests were used to illustrate differences in RFS and OS according to CD163 and CD68 expression. Cox regression proportional hazards models were used to estimate hazard ratios (HR) for death from breast cancer according to CD68 and CD163 expression in both uni- and multivariate analysis. Covariates with a P value ≤ 0.05 in the univariate analysis were included in the multivariate analysis. All statistical tests were two-sided and P ≤ 0.05 was considered significant. Statistical analysis was performed using IBM SPSS Statistics 25 (IBM, Armonk, NY, USA).
Results
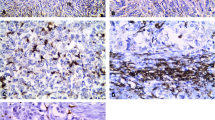
The CD68 and CD163 expressions in TS and TN were determined for all 107 samples. CD68+ (Fig. 1a, b) and CD163+ (Fig. 1c, d) macrophages were detected in both the TS and TN of TNC. The relationship between the density of TAMs (CD68+, or CD163+) and clinicopathological features is shown in Table 2. The study demonstrated that a high density of CD68+ TAMs in both TS and TN was significantly associated with larger tumor size (p = 0.036; p = 0.004). Whereas a high density of CD163+ TAMs in TN was also significantly related to larger tumor size (p = 0.002), however, not in TS (p = 0.634). Moreover, a high density of CD163+ TAMs in both TS and TN were correlated with higher histological grade (p < 0.001; p = 0.010), higher recurrence rate (p < 0.001; p = 0.004), and higher breast cancer mortality (p = 0.004, p = 0.012). In contrast, no significant correlations were found between the infiltration densities of TAMs (CD68+, CD163+, CD163/CD68 ratio) and TILs in both TS (p = 0.635, p = 0.382, and p = 0.382, respectively) or TN (p = 0.635, p = 0.861, and p = 0.670, respectively). No correlation was found between the CD163/CD68 ratios for either TS or TN or in terms of clinicopathological features.
Immunohistochemical staining for the infiltration of CD68+ tumor-associated macrophages (TAMs) and CD163+ TAMs in triple-negative cancer (TNC) of the breast. Representative images of high density CD68+ staining (a, b) and CD163+ staining (c, d) in tumor stroma and tumor nest. (original magnification, ×200)
Univariate and multivariate Cox regression analysis of RFS and OS were performed using clinicopathological prognostic factors and expressions of CD68 and CD163 (Table 3). Multivariate Cox regression analyses revealed that age and CD163+ TAMs in both TS and TN were independent prognostic factors for RFS (HR = 0.164, 95% CI 0.048–0.560, p = 0.004; HR = 9.059, 95% CI 1.160–70.76, p = 0.036; HR = 4.476, 95% CI 1.028–22.08, p = 0.046) and OS (HR = 0.095, 95% CI 0.024–0.374, p = 0.001; HR = 10.69, 95% CI 1.313–87.18, p = 0.027; HR = 5.017, 95% CI 1.065–23.64, p = 0.041).
We investigated survival rate with regard to the different expressions of TAMs status using the Kaplan-Meier method and log-rank test. No correlation was found in the higher CD68+ TAMs density in both TS and TN with RFS (p = 0.119; p = 0.957) or OS (p = 0.104; p = 0.911) (Fig. 2a, b, c, and d). A higher CD163+ TAMs density in both TS and TN was correlated with unfavorable RFS (p = 0.003; p = 0.022) and OS (p = 0.005; p = 0.026) (Fig. 2e–h). However, no correlation was identified between high CD163/CD68+ ratios in both TS and TN with RFS (p = 0.085, p = 0.782) or OS (p = 0.102, p = 0.891) (Fig. 2i–l).
Prognostic significance of TAMs in breast cancer. Kaplan–Meier curves for relapse-free survival (RFS) and overall survival (OS) were stratified by the median values as the cut-off for prognostic evaluation and divided into low or high TAMs variable subsets. CD68+ TAMs did not demonstrate prognostic significance for RFS (a, c) or OS (b, d) in tumor stroma (TS) and tumor nest (TN). High density of CD163+ TAMs in TS and TN were associated with poor RFS (e, g) and OS (f, h). The RFS (i, k) and OS (j, l) curves according to the infiltration density of CD163/CD68+ ratios in TS and TN
Discussion
TAMs can contribute to tumor destruction and influence tumor growth and progression. M1-like macrophages, characterized by CD68 expression, produce free radicals that can lead to DNA damage with the potential to contribute to tumoricidal activity. In contrast, M2-like macrophages, characterized by both CD68 and CD163 expression, are considered to promote tumor growth and metastasis by releasing chemokines, which are inflammatory growth factors [1,2,3]. Even so, the prognostic significance of localizations and densities of CD68+ and CD163+ TAMs in TNC is not well understood.
In our study of TNC, no correlation was found between CD68+ TAMs in TS and TN with any clinicopathological findings, OS or RFS by univariate analysis. CD68 is a pan-macrophage marker as it stains both M1-like and M2-like TAMs. Controversy remains over the role of CD68 in cancer. CD68+ TAMs correlated with favorable prognosis in several organs, such as prostate [20], lung [21], and brain tumors [22]. In contrast, poor prognosis was reported in uterine cervix [23], and bladder carcinomas [24]. Furthermore, earlier studies report that a high density of CD68+ TAMs infiltration in invasive breast cancer was associated with higher vascularity and nodal metastasis, as well as reduced RFS and OS [25, 26]. Also, Tsutsui et al. reported that a high density of CD68+ TAMs had significantly worse disease-free survival [18]. Further, Mahmoud et al. reported on CD68+ TAMs using a large cohort of patients. In their univariate analysis, a high density of CD68+ TAMs predicted worse breast cancer specific survival and shorter disease-free interval [27]. These results suggest that CD68+ TAMs induce an immune response that supports tumor invasion. However, similar to our findings, Medrek et al. found that CD68+ TAMs showed no correlations between clinicopathological findings and RFS and OS in TNC [28]. Recently, Yang et al. reported that CD68+ TAMs in TNC were not associated with RFS or OS in multivariate analysis [7]. These results suggest that CD68+ TAMs are not an important prognostic factor for patients; however, these results are probably due to CD68 expressing both M1-like and M2-like TAMs, which have opposing effects.
CD163, a well-known specific marker for M2-like macrophages, was found to be closely correlated with unfavorable prognostic factors in several studies [8,9,10, 29, 30]. Medrek et al. reported that TNC showed more TAMs infiltration, especially CD163+ cells, than other types of breast cancers [28]. However, they did not find any prognostic significance of CD163+ TAMs in TN. Further, Yang et al. found that increased CD163+ TAMs in TS were correlated with unfavorable clinicopathological factors, and worse RFS and OS [7]. However, they did not find any statistical difference in CD163+ TAMs in TN. Several studies in breast cancer have reported the locations of TAMs [7, 19, 27, 28]. Therefore, we also used full block-face tissue sections to estimate TAMs in TS and TN separately to assess their prognostic value in our TNC cohort. We found in multivariate Cox regression analyses using the median as the cut-off that CD163+ TAMs in both TS and TN were independent prognostic factors for worse RFS and OS. From these results, it is suggested that CD163+ TAMs affect the prognosis of TNC by not only regulating the immune reaction by TAMs in TS, but also through their direct influence on TN.
We also examined the correlation between TAMs and TILs which have recently been highlighted as prognostic markers and potential targets for adjuvant therapy [31,32,33,34]. TILs have antitumor activity and a favorable prognostic effect in breast cancer, especially in TNC [16, 35,36,37]. In our study, no significant correlations were found between the infiltration densities of TAMs and TILs. However, we could not draw any conclusion on the basis of our small number of cases.
There is a limitation in this study. First, the methods and subtypes of breast cancer patients were different in other studies, including our own. Yang et al. examined cases in which Basal-like carcinoma was defined by not only TNC, but also by EGFR and/or CK5/6 expression [7]. Second, although CD163 is regarded as a highly specific M2 macrophage marker, it can also be expressed by myeloid dendritic cells (MDCs). Both macrophages and MDCs are members of the mononuclear phagocyte system, these cells are considered distinct cell types based on their morphology and functions. Macrophages are defined as large vacuolar cells that have oval or rounded nuclei, while MDCs are characterized as stellate migratory cells. Therefore, we could have excluded the majority of the CD68+ and CD163+ MDCs with morphological features. Nevertheless, it could not be confirmed whether or not CD68+ and CD163+ MDCs are located in TS and TN. Of the different cell characteristics, surface markers are often used to distinguish MDCs from macrophages, but phenotypic analysis has been considered insufficient to define MDCs subsets. Some specific markers have been suggested to detect M1/M2 macrophages, but they remain controversial. In the future, more studies on larger sample sizes and TAMs labeling new, reliable macrophage markers are needed to evaluate the clinical value. Further, Medrek et al. observed some CD163+ areas that lacked CD68 expression. They suggested this result was due to a CD163-expressing subset of immature myeloid cells with prognostic impact [28]. Here, we confirmed TAMs not only by immunohistochemical staining, but also H&E staining, then estimated the number of typical macrophages and excluded the possibility that MDCs cells or myeloid-derived cells expressed CD163. However, further investigation is needed to identify TAMs’ roles in TNC with new, specific markers in future studies.
Conclusions
We examined the prognostic value of TAMs in TNC. Our study found that infiltration of CD163+ TAMs, rather than CD68, in both TS and TN was associated with poor prognosis in TNC patients by multivariate analysis. This suggested that CD163+ TAMs may affect the prognosis of TNC by not only regulating the immune reaction by TAMs in TS, but also through their direct influence on TN.
References
Murray PJ, Wynn TA (2011) Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol 89:557–563. https://doi.org/10.1189/jlb.0710409
Solinas G, Germano G, Mantovani A, Allavena P (2009)Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86:1065–1073. https://doi.org/10.1189/jlb.0609385
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35. https://doi.org/10.1038/nri978
Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, Wei LX (2014)Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-inducedepithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett 352:160–168. https://doi.org/10.1016/j.canlet.2014.05.008
Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, Xu H, Xu H (2017) The prognostic and Clinicopathological significance of tumor-associated macrophages in patients with gastric Cancer: a meta-analysis. PLoS One 12:e0170042. https://doi.org/10.1371/journal.pone.0170042
Cao L, Che X, Qiu X, Li Z, Yang B, Wang S, Hou K, Fan Y, Qu X, Liu Y (2019) M2 macrophage infiltration into tumor islets leads to poor prognosis in non-small-cell lung cancer. Cancer Manag Res 11:6125–6138. https://doi.org/10.2147/CMAR.S199832
Yang M, Li Z, Ren M, Li S, Zhang L, Zhang X, Liu F (2018) Stromal infiltration of tumor-associated macrophages conferring poor prognosis of patients with basal-like breast carcinoma. J Cancer 9:2308–2316. https://doi.org/10.7150/jca.25155
Ni C, Yang L, Xu Q, Yuan H, Wang W, Xia W, Gong D, Zhang W, Yu K (2019)CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: a retrospective study and meta-analysis. J Cancer 10:4463–4472. https://doi.org/10.7150/jca.33914
Zhao X, Qu J, Sun Y et al (2017) Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget 8:30576–30586. https://doi.org/10.18632/oncotarget.15736
Tiainen S, Tumelius R, Rilla K, Hämäläinen K, Tammi M, Tammi R, Kosma VM, Oikari S, Auvinen P (2015) High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology. 66:873–883. https://doi.org/10.1111/his.12607
Mersin H, Yildirim E, Berberoglu U, Gulben K (2008) The prognostic importance of triple negative breast carcinoma. Breast. 17:341–346. https://doi.org/10.1016/j.breast.2007.11.031
Sorlie T, Tibshirani R, Parker J et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 100:8418–8423. https://doi.org/10.1073/pnas.0932692100
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, Perou CM, Regan MM, Rimm DL, Symmans WF, Torlakovic EE, Varella L, Viale G, Weisberg TF, McShane LM, Wolff AC (2020) Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J Clin Oncol 38:1346–1366. https://doi.org/10.1200/JCO.19.02309
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142:1364–1382. https://doi.org/10.5858/arpa.2018-0902-SA
Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, van den Eynden GGGM, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SR, Schnitt S, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O’Toole S, Larsimont D, de Wind R, Curigliano G, André F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB (2017) Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international Immunooncology biomarkers working group: part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, Metastatic Tumor Deposits and Areas for Further Research. Adv Anat Pathol 24:235–251. https://doi.org/10.1097/PAP.0000000000000162
Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria S, Gray R, Munzone E, Lemonnier J, Sotiriou C, Piccart MJ, Kellokumpu-Lehtinen PL, Vingiani A, Gray K, Andre F, Denkert C, Salgado R, Michiels S (2019)Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol 7:559–569. https://doi.org/10.1200/JCO.18.01010
Vicioso L, Gonzalez FJ, Alvarez M, Ribelles N, Molina M, Marquez A, Perez L, Matilla A, Alba E (2006) Elevated serum levels of vascular endothelial growth factor are associated with tumor-associated macrophages in primary breast cancer. Am J Clin Pathol 125:111–118. https://doi.org/10.1309/0864AF2U3LGPCF3J
Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S (2005) Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 14:425–431
Ch’ng E, Tuan Sharif S, Jaafar H (2013) In human invasive breast ductal carcinoma, tumor stromal macrophages and tumor nest macrophages have distinct relationships with clinicopathological parameters and tumor angiogenesis. Virchows Arch 462:257–267. https://doi.org/10.1007/s00428-012-1362-4
Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A (2000) Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 17:445–451. https://doi.org/10.3892/ijo.17.3.445
Kerr KM, Johnson SK, King G, Kennedy MM, Weir J, Jeffrey R (1998) Partial regression in primary carcinoma of the lung: does it occur? Histopathology. 33:55–63
Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST (1997) Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol 93:518–527. https://doi.org/10.1007/s004010050647
Riethdorf L, Riethdorf S, Gutzlaff K et al (1996) Differential expression of the monocyte chemoattractant protein-1 gene in human papillomavirus-16-infected squamous intraepithelial lesions and squamous cell carcinomas of the cervix uteri. Am J Pathol 149:1469–1476
Bostrom MM, Irjala H, Mirtti T et al (2015)Tumor-associated macrophages provide significant prognostic information in Urothelial bladder Cancer. PLoS One 10:e0133552. https://doi.org/10.1371/journal.pone.0133552
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56:4625–4629
Jubb AM, Soilleux EJ, Turley H et al (2010) Expression of vascular notch Ligand Delta-like 4 and inflammatory markers in breast Cancer. Am. J. Pathol 176:2019–2028. https://doi.org/10.2353/ajpath.2010.090908
Mahmoud SM, Lee AH, Paish EC et al (2012)Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol 65:159–163. https://doi.org/10.1136/jclinpath-2011-200355
Medrek C, Ponten F, Jirstrom K, Leandersson K (2012) The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12:306. https://doi.org/10.1186/1471-2407-12-306
Sousa S, Brion R, Lintunen M, Kronqvist P, Sandholm J, Mönkkönen J, Kellokumpu-Lehtinen PL, Lauttia S, Tynninen O, Joensuu H, Heymann D, Määttä JA (2015) Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res 17:101–110. https://doi.org/10.1186/s13058-015-0621-0
Nguyen TT, Schwartz EJ, West RB et al (2005) Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol 29:617–624 00000478-200505000-00007
Tang X (2013)Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett 332:3–10. https://doi.org/10.1016/j.canlet.2013.01.024
Xuan QJ, Wang JX, Nanding A, Wang ZP, Liu H, Lian X, Zhang QY (2014)Tumor-associated macrophages are correlated with tamoxifen resistance in the postmenopausal breast cancer patients. Pathol Oncol Res 20:619–624. https://doi.org/10.1007/s12253-013-9740-z
Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Törne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G (2010)Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28:105–113. https://doi.org/10.1200/JCO.2009.23.7370
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, van Eenoo F, Rouas G, Francis P, Crown JPA, Hitre E, de Azambuja E, Quinaux E, di Leo A, Michiels S, Piccart MJ, Sotiriou C (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31:860–867. https://doi.org/10.1200/JCO.2011.41.0902
Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, Katsumata N, Kinoshita T, Takiguchi Y, Tanzawa H, Fujiwara Y (2012)Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 132:793–805. https://doi.org/10.1007/s10549-011-1554-7
Adams S, Gray RJ, Demaria S et al (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32:2959–2966 JCO.2013.55.0491
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, Piccart MJ, Loibl S, Denkert C, Smyth MJ, Joensuu H, Sotiriou C (2014) Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 25:1544–1550. https://doi.org/10.1093/annonc/mdu112
Acknowledgments
The authors thank C Matsuyama and A Shimizu for their advice and technical assistance with IHC staining.
Author information
Authors and Affiliations
Contributions
T Jamiyan and H Kuroda contributed equally to this work; A Abe and M Hayashi collected clinical information; T Jamiyan and Kuroda H reviewed the pathological diagnosis; T Jamiyan and H Kuroda analyzed the data and wrote the manuscript; R Yamaguchi made critical revisions to the manuscript; T Jamiyan and H Kuroda designed the study; H Kuroda gave the final approval of the manuscript for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The authors adhered to institutional ethical standards.
Conflict of interest
The authors declare no potential competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jamiyan, T., Kuroda, H., Yamaguchi, R. et al. CD68- and CD163-positive tumor-associated macrophages in triple negative cancer of the breast. Virchows Arch 477, 767–775 (2020). https://doi.org/10.1007/s00428-020-02855-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02855-z