Abstract
Vascular malformations (VMs) are rare congenital anomalies that develop during embryogenesis in different types of vessels. Several triggering factors of cutaneous VMs include trauma, infections, or hormonal changes. We investigated the expression of hormonal receptors (androgen, estrogen, progesterone) in tissue samples of well-characterized VMs. A secondary objective was to identify self-reported triggering factors for these VMs, including hormonal changes, in the cohort of patients. We included patients with VM samples obtained in the tertiary center for vascular anomalies of the University Hospital Center of Tours, France, from January 1, 2007, to August 1, 2018. Immunohistochemistry was used to detect the expression of hormonal receptors (estrogen, progesterone, androgens). We obtained 51 samples from 51 patients: 13 cystic lymphatic malformations (CLMs), 16 venous malformations (VeMs), 11 arteriovenous malformations (AVMs), 4 combined VMs, 4 PIK3CA-related overgrowth spectrum, 1 Parkes-Weber syndrome, 1 Gorham syndrome, and 1 multiple lymphangioendotheliomatosis with thrombopenia. In total, 38 (74.5%) samples were positive for androgen receptor: 11 (84.6%) CLMs, 12 (75.0%) VeMs, 8 (72.2%) AVMs, and 7/11 (63.5%) other samples. All samples were negative for estrogen and progesterone receptors. Triggering factors were self-reported in 7 cases and were most frequently hormonal changes (n = 6, 18.2%). Hormonal triggers were frequent in AVMs (n = 4). Among patients with identified hormonal triggers, VM samples were positive for androgen receptor in 3 and negative in 3. Three-quarters of our VM samples expressed androgen receptor, and most CLM, VeM, and AVM samples were positive. Hormonal triggers were identified in 6/33 patients, mostly with AVMs.

Similar content being viewed by others
Change history
19 March 2019
The original version of this article contained error. Table 2 was shown in the wrong version, thus corrected table is shown in this article.
References
Cox JA, Bartlett E, Lee EI (2014) Vascular malformations: a review. Semin Plast Surg 28(2):58–63
Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, Burrows P, Frieden IJ, Garzon MC, Lopez-Gutierrez JC, Lord DJE, Mitchel S, Powell J, Prendiville J, Vikkula M, on behalf of the ISSVA Board and Scientific Committee (2015) Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 136(1):e203–e214
Macmurdo CF, Wooderchak-Donahue W, Bayrak-Toydemir P, Le J, Wallenstein MB, Milla C et al (2016) RASA1 somatic mutation and variable expressivity in capillary malformation/arteriovenous malformation (CM/AVM) syndrome. Am J Med Genet A 170(6):1450–1454
Amyere M, Revencu N, Helaers R, Pairet E, Baselga E, Cordisco M, Chung W, Dubois J, Lacour JP, Martorell L, Mazereeuw-Hautier J, Pyeritz RE, Amor DJ, Bisdorff A, Blei F, Bombei H, Dompmartin A, Brooks D, Dupont J, González-Enseñat MA, Frieden I, Gérard M, Kvarnung M, Hanson-Kahn AK, Hudgins L, Léauté-Labrèze C, McCuaig C, Metry D, Parent P, Paul C, Petit F, Phan A, Quere I, Salhi A, Turner A, Vabres P, Vicente A, Wargon O, Watanabe S, Weibel L, Wilson A, Willing M, Mulliken JB, Boon LM, Vikkula M (2017) Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation. 136(11):1037–1048
Kurek KC, Luks VL, Ayturk UM, Alomari AI, Fishman SJ, Spencer SA, Mulliken JB, Bowen ME, Yamamoto GL, Kozakewich HPW, Warman ML (2012) Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am J Hum Genet 90(6):1108–1115
Martins L, Giovani PA, Rebouças PD, Brasil DM, Haiter Neto F, Coletta RD, Machado RA, Puppin-Rontani RM, Nociti Jr FH, Kantovitz KR (2017) Computational analysis for GNAQ mutations: new insights on the molecular etiology of Sturge-Weber syndrome. J Mol Graph Model 76:429–440
Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, North PE, Marchuk DA, Comi AM, Pevsner J (2013) Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med 368(21):1971–1979
Soblet J, Limaye N, Uebelhoer M, Boon LM, Vikkula M (2013) Variable somatic TIE2 mutations in half of sporadic venous malformations. Mol Syndromol 4(4):179–183
Limaye N, Kangas J, Mendola A, Godfraind C, Schlögel MJ, Helaers R, Eklund L, Boon LM, Vikkula M (2015) Somatic activating PIK3CA mutations cause venous malformation. Am J Hum Genet 97(6):914–921
Vikkula M, Boon LM, Carraway KL, Calvert JT, Diamonti AJ, Goumnerov B et al (1996) Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 87(7):1181–1190
Keppler-Noreuil KM, Rios JJ, Parker VER, Semple RK, Lindhurst MJ, Sapp JC, Alomari A, Ezaki M, Dobyns W, Biesecker LG (2015) PIK3CA-related overgrowth spectrum (PROS): diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A 167A(2):287–295
Couto JA, Huang AY, Konczyk DJ, Goss JA, Fishman SJ, Mulliken JB, Warman ML, Greene AK (2017) Somatic MAP2K1 mutations are associated with extracranial arteriovenous malformation. Am J Hum Genet 100(3):546–554
Adams DM, Trenor CC, Hammill AM, Vinks AA, Patel MN, Chaudry G, Wentzel MS, Mobberley-Schuman PS, Campbell LM, Brookbank C, Gupta A, Chute C, Eile J, McKenna J, Merrow AC, Fei L, Hornung L, Seid M, Dasgupta AR, Dickie BH, Elluru RG, Lucky AW, Weiss B, Azizkhan RG (2016) Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 137(2):e20153257
Vogelzang RL, Atassi R, Vouche M, Resnick S, Salem R (2014) Ethanol embolotherapy of vascular malformations: clinical outcomes at a single center. J Vasc Interv Radiol 25(2):206–213 quiz 214
Lee JW, Chung HY, Cerrati EW, TM O, Waner M (2015) The natural history of soft tissue hypertrophy, bony hypertrophy, and nodule formation in patients with untreated head and neck capillary malformations. Dermatol Surg 41(11):1241–1245
Hassanein AH, Mulliken JB, Fishman SJ, Quatrano NA, Zurakowski D, Greene AK (2012) Lymphatic malformation: risk of progression during childhood and adolescence. J Craniofac Surg 23(1):149–152
Hassanein AH, Mulliken JB, Fishman SJ, Alomari AI, Zurakowski D, Greene AK (2012) Venous malformation: risk of progression during childhood and adolescence. Ann Plast Surg 68(2):198–201
Liu AS, Mulliken JB, Zurakowski D, Fishman SJ, Greene AK (2010) Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg 125(4):1185–1194
Fanous AA, Jowdy PK, Lipinski LJ, Balos LL, Li V (2016) Association between trauma and acute hemorrhage of cavernous malformations in children: report of 3 cases. J Neurosurg Pediatr 18(3):263–268
Talay S, Erkut B, Kabalar ME (2012) Surgical treatment of a case with rapidly growing mass lesion after trauma: on the left forearm arteriovenous malformation. Ulus Travma Acil Cerrahi Derg 18(4):344–346
Quack Loetscher KC, Jandali A-R, Garzoli E, Pok J, Beinder E (2005) Axillary cavernous lymphangioma in pregnancy and puerperium. Gynecol Obstet Investig 60(2):108–111
Elliott JA, Rankin RN, Inwood MJ, Milne JK (1985) An arteriovenous malformation in pregnancy: a case report and review of the literature. Am J Obstet Gynecol 152(1):85–88
Fishman A, Paldi E (1989) Klippel-Trenaunay syndrome with complications during pregnancy. Harefuah. 116(3):147–148
Mcculley S, Fourie L, Hull SM (1997) Spontaneous digital arteriovenous malformation in a 28-year-old pregnant female. Br J Dermatol 136(3):472–473
Liu W, Zhang S, Hu T, Jiang X, Hu X, Feng J (1999) Sex hormone receptor of hemangioma and vascular malformation in children. Zhonghua Wai Ke Za Zhi 37(5):295–297
Kulungowski AM, Hassanein AH, Nosé V, Fishman SJ, Mulliken JB, Upton J et al (2012) Expression of androgen, estrogen, progesterone, and growth hormone receptors in vascular malformations. Plast Reconstr Surg 129(6):919e–924e
Wilson CM, McPhaul MJ (1996) A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol 120(1):51–57
Bläuer M, Vaalasti A, Pauli SL, Ylikomi T, Joensuu T, Tuohimaa P (1991) Location of androgen receptor in human skin. J Invest Dermatol 97(2):264–268
Lehmann BD, Bauer JA, Schafer JM, Pendleton CS, Tang L, Johnson KC, Chen X, Balko JM, Gómez H, Arteaga CL, Mills GB, Sanders ME, Pietenpol JA (2014) PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res 16(4):406
Venot Q, Blanc T, Rabia SH, Berteloot L, Ladraa S, Duong J-P, Blanc E, Johnson SC, Hoguin C, Boccara O, Sarnacki S, Boddaert N, Pannier S, Martinez F, Magassa S, Yamaguchi J, Knebelmann B, Merville P, Grenier N, Joly D, Cormier-Daire V, Michot C, Bole-Feysot C, Picard A, Soupre V, Lyonnet S, Sadoine J, Slimani L, Chaussain C, Laroche-Raynaud C, Guibaud L, Broissand C, Amiel J, Legendre C, Terzi F, Canaud G (2018) Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature. 558(7711):540–546
Duyka LJ, Fan CY, Coviello-Malle JM, Buckmiller L, Suen JY (2009) Progesterone receptors identified in vascular malformations of the head and neck. Otolaryngol Head Neck Surg 141(4):491–495
Hiort O (2002) Androgens and puberty. Best Pract Res Clin Endocrinol Metab 16(1):31–41
Sidiropoulou E, Ghizzoni L, Mastorakos G (2000) Adrenal Androgens. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM et al (eds) Endotext. MDText.com, Inc., South Dartmouth
Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY (2005) Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev 26(1):114–146
Maclellan RA, Vivero MP, Purcell P, Kozakewich HP, DiVasta AD, Mulliken JB et al (2014) Expression of follicle-stimulating hormone receptor in vascular anomalies. Plast Reconstr Surg 133(3):344e–351e
Acknowledgements
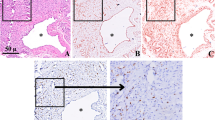
We thank Dr. M. Dorbeau for the H&E images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: Table 2 was shown in the wrong version, thus corrected table is shown in this article.
Rights and permissions
About this article
Cite this article
Ventéjou, S., Machet, MC., Herbreteau, D. et al. Hormonal receptors in cutaneous vascular malformations: 51 cases. Virchows Arch 474, 755–761 (2019). https://doi.org/10.1007/s00428-019-02546-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02546-4