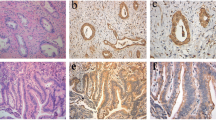
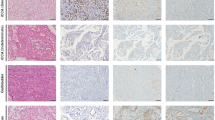
Abstract
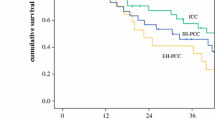
Intrahepatic cholangiocarcinoma (ICC) has universally poor outcome, mainly due to its late clinical presentation. Identification of specific biomarkers and development of effective treatment are still urgently required. Mutations in PBRM-1 and BAP-1 genes, and the expression of S100P have been related to survival in ICC. miR-31 seems also to play important regulatory functions in ICC and it directly regulates BAP-1 expression in lung cancer. In this study, tissue expression of BAP-1, PBRM-1, S100P, and miR-31 was investigated in ICC and correlated with clinical-pathological features. Sixty-one consecutive patients who underwent curative hepatic resection for ICC were enrolled. None received any therapy prior to surgery. Immunostaining for BAP-1, PBRM-1, and S100P, and in situ hybridization for miR-31 were performed, using tissue microarray slides. A strong retained expression of BAP-1 and PBRM-1 was associated with a reduced overall (p = 0.04 and p = 0.002, respectively) and disease-free survival (p = 0.05 and p = 0.02, respectively). An overexpression of S100P was related to a reduced overall survival (p = 0.005). The multivariate analyses identified the presence of perineural invasion and the retained PBRM-1 expression as independent predictors of worse overall [p = 0.02, hazard ratio (HR) = 2.25 (1.16–4.39) and p = 0.001, HR = 3.13 (1.56–6.28), respectively] and disease-free survivals [p = 0.03, HR = 2.43 (1.09–5.4) and p = 0.03, HR = 2.51 (1.11–5.67), respectively]. An overexpression of S100P was predictive of a worse overall survival [p = 0.02, HR = 1.66 (1.08–2.55)]. High levels of miR-31 were significantly associated to a low expression of BAP-1 protein (p = 0.03). In ICC, a retained expression of BAP-1 and PBRM-1, and an overexpression of S100P are related to a poor prognosis.



Similar content being viewed by others
References
Squadroni M, Tondulli L, Gatta G, Mosconi S, Beretta G, Labianca R (2017) Cholangiocarcinoma. Crit Rev Oncol Hematol 116:11–31
Bartella I, Dufour JF (2015) Clinical diagnosis and staging of intrahepatic cholangiocarcinoma. J Gastrointestin Liver Dis 24:481–489
Kayhanian H, Smyth EC, Braconi C (2017) Emerging molecular targets and therapy for cholangiocarcinoma. World J Gastrointest Oncol 9:268–280
Brandi G, Venturi M, Pantaleo MA, Ercolani G, GICO (2016) Cholangiocarcinoma: current opinion on clinical practice diagnostic and therapeutic algorithms. A review of the literature and a long-standing experience of a referral center. Dig Liver Dis 48:231–241
Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, Zuo M, Zinner R, Hong D, Javle M et al (2014) Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 9:e115383
Haga H, Patel T (2015) Molecular diagnosis of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 22:114–123
Ruzzenente A, Fassan M, Conci S, Simbolo M, Lawlor RT, Pedrazzani C, Capelli P, D'Onofrio M, Iacono C, Scarpa A, Guglielmi A (2016) Cholangiocarcinoma heterogeneity revealed by multigene mutational profiling: clinical and prognostic relevance in surgically resected patients. Ann Surg Oncol 23:1699–1707
Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Wood LD et al (2013) Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 45:1470–1473
Piva F, Giulietti M, Occhipinti G, Santoni M, Massari F, Sotte V, Iacovelli R, Burattini L, Santini D, Montironi R, Cascinu S, Principato G (2015) Computational analysis of the mutations in BAP1, PBRM1 and SETD2 genes reveals the impaired molecular processes in renal cell carcinoma. Oncotarget 6:32161–32168
Ventii KH, Devi NS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, Wilkinson KD (2008) BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res 68:6953–6962
Wang A, Papneja A, Hyrcza M, Al-Habeeb A, Ghazarian D (2016) Gene of the month: BAP1. J Clin Pathol 69:750–753
Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G (2013) BAP1 and cancer. Nat Rev Cancer 13:153–159
Luchini C, Robertson SA, Hong SM, Felsenstein M, Anders RA, Pea A, Nottegar A, Veronese N, He J, Weiss MJ, Capelli P, Scarpa A, Argani P, Kapur P, Wood LD (2017) PBRM1 loss is a late event during the development of cholangiocarcinoma. Histopathology 71:375–382
Cigognetti M, Lonardi S, Fisogni S, Balzarini P, Pellegrini V, Tironi A, Bercich L, Bugatti M, Rossi G, Murer B, Barbareschi M, Giuliani S, Cavazza A, Marchetti G, Vermi W, Facchetti F (2015) BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 28:1043–1057
Misumi K, Hayashi A, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, Kokudo N, Fukayama M (2017) Intrahepatic cholangiocarcinoma frequently shows loss of BAP1 and PBRM1 expression, and demonstrates specific clinicopathological and genetic characteristics with BAP1 loss. Histopathology 70:766–774
Hu C, Huang F, Deng G, Nie W, Huang W, Zeng X (2013) miR-31 promotes oncogenesis in intrahepatic cholangiocarcinoma cells via the direct suppression of RASA1. Exp Ther Med 6:1265–1270
Yu M, Liang H, Fu Z, Wang X, Liao Z, Zhou Y, Liu Y, Wang Y, Hong Y, Chen X et al (2016) BAP1 suppresses lung cancer progression and is inhibited by miR-31. Oncotarget 7:13742–13753
Aishima S, Oda Y (2015) Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci 22:94–100
Sato Y, Harada K, Sasaki M, Nakanuma Y (2013) Clinicopathological significance of S100 protein expression in cholangiocarcinoma. J Gastroenterol Hepatol 28:1422–1429
Aishima S, Fujita N, Mano Y, Kubo Y, Tanaka Y, Taketomi A, Shirabe K, Maehara Y, Oda Y (2011) Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J Surg Pathol 35:590–598
Tsai JH, Huang WC, Kuo KT, Yuan RH, Chen YL, Jeng YM (2012) S100P immunostaining identifies a subset of peripheral-type intrahepatic cholangiocarcinomas with morphological and molecular features similar to those of perihilar and extrahepatic cholangiocarcinomas. Histopathology 61:1106–1116
Nakanuma Y, Kakuda Y (2015) Pathologic classification of cholangiocarcinoma: new concepts. Best Pract Res Clin Gastroenterol 29:277–293
Saraggi D, Galuppini F, Remo A, Urso EDL, Bacchin D, Salmaso R, Lanza C, Bao RQ, Fanelli GN, Guzzardo V, Luchini C, Scarpa M, Farinati F, Fassan M, Rugge M (2017) PD-L1 overexpression in ampulla of Vater carcinoma and its pre-invasive lesions. Histopathology 71:470–474
Lovat F, Fassan M, Gasparini P, Rizzotto L, Cascione L, Pizzi M, Vicentini C, Balatti V, Palmieri D, Costinean S, Croce CM (2015) miR-15b/16-2 deletion promotes B-cell malignancies. Proc Natl Acad Sci USA 112:11636–11641
Andrici J, Goeppert B, Sioson L, Clarkson A, Renner M, Stenzinger A, Tayao M, Watson N, Farzin M, Gill AJ et al (2016) Loss of BAP1 expression occurs frequently in intrahepatic cholangiocarcinoma. Medicine (Baltimore) 95:e2491
Nakaoka T, Saito Y, Saito H (2017) Aberrant DNA methylation as a biomarker and a therapeutic target of cholangiocarcinoma. Int J Mol Sci 18:E1111
Chowdhury B, Porter EG, Stewart JC, Ferreira CR, Schipma MJ, Dykhuizen EC (2016) PBRM1 regulates the expression of genes involved in metabolism and cell adhesion in renal clear cell carcinoma. PLoS One 11:e0153718
Cardinale V, Bragazzi MC, Carpino G, Torrice A, Fraveto A, Gentile R, Pasqualino V, Melandro F, Aliberti C, Bastianelli C, Brunelli R, Berloco PB, Gaudio E, Alvaro D (2013) Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr 2:272–280
Author information
Authors and Affiliations
Contributions
Samantha Sarcognato: conception and design, acquisition of data, analysis and interpretation of data, writing and revision of the manuscript;
Enrico Gringeri: conception and design, acquisition of data, analysis and interpretation of data, writing and revision of the manuscript;
Matteo Fassan: conception and design, development of methodology, acquisition of data, analysis and interpretation of data, writing and revision of the manuscript;
Michela Di Giunta: acquisition of data, analysis and interpretation of data;
Valeria Maffeis: analysis and interpretation of data;
Vincenza Guzzardo: development of methodology, technical and material support;
Umberto Cillo: conception and design, interpretation of data, revision of the manuscript;
Maria Guido: conception and design, analysis and interpretation of data, writing and revision of the manuscript, study supervision.
All authors gave final approval for publication.
Maria Guido takes full responsibility for the work as a whole, including the study design, access to data and the decision to submit and publish the manuscript.
Corresponding author
Ethics declarations
The study complies with the ethical guidelines of the 1975 Declaration of Helsinki and obtained the approval from the local Ethics Committee (Ethics Committee for Clinical Research—University Hospital of Padova, Italy; protocol #: 0038038/2017).
Informed consent
All the patients gave their appropriate informed consent to the surgical procedure.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical responsibilities of authors
All of the authors confirm that each of them qualifies for every one of the 4 criteria of authorship.
Electronic supplementary material
Supplementary Fig. 1
An example of a negative (A, original magnification 10x) and a positive (B, original magnification 10x) PBRM-1 reaction in intrahepatic cholangiocarcinoma (PNG 743 kb)
Supplementary Fig. 2
A significantly reduced overall survival was observed in cases with an overexpression of S100P, as shown by the Kaplan-Meier curve (PNG 106 kb)
Rights and permissions
About this article
Cite this article
Sarcognato, S., Gringeri, E., Fassan, M. et al. Prognostic role of BAP-1 and PBRM-1 expression in intrahepatic cholangiocarcinoma. Virchows Arch 474, 29–37 (2019). https://doi.org/10.1007/s00428-018-2478-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2478-y